
RHEUMATOID ARTHRITIS
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease characterised by joint inflammation, with consequent tissue damage and destruction that causes pain, deformity and disability to those affected. Patients are treated with painkillers and a range of disease-modifying anti-rheumatic drugs (DMARDs), the trend being for older generic small molecules at first-line moving to newer biologic drugs as disease severity progresses. The market for RA drugs represents one of the highest value pharmaceutical markets. Specifically, the growth and large size of the market have been driven by revenues from novel, highly priced, biologic drugs. The first biologic approved for the treatment of RA was Amgen’s anti-TNF fusion protein Enbrel® (etanercept) in 1998 (US) and since that time a number of others have reached the market, their combined sales hitting nearly $40 billion in 2015. Biologic drugs currently dominate the market, so there is certainly space to innovate with advanced therapies and small molecules.
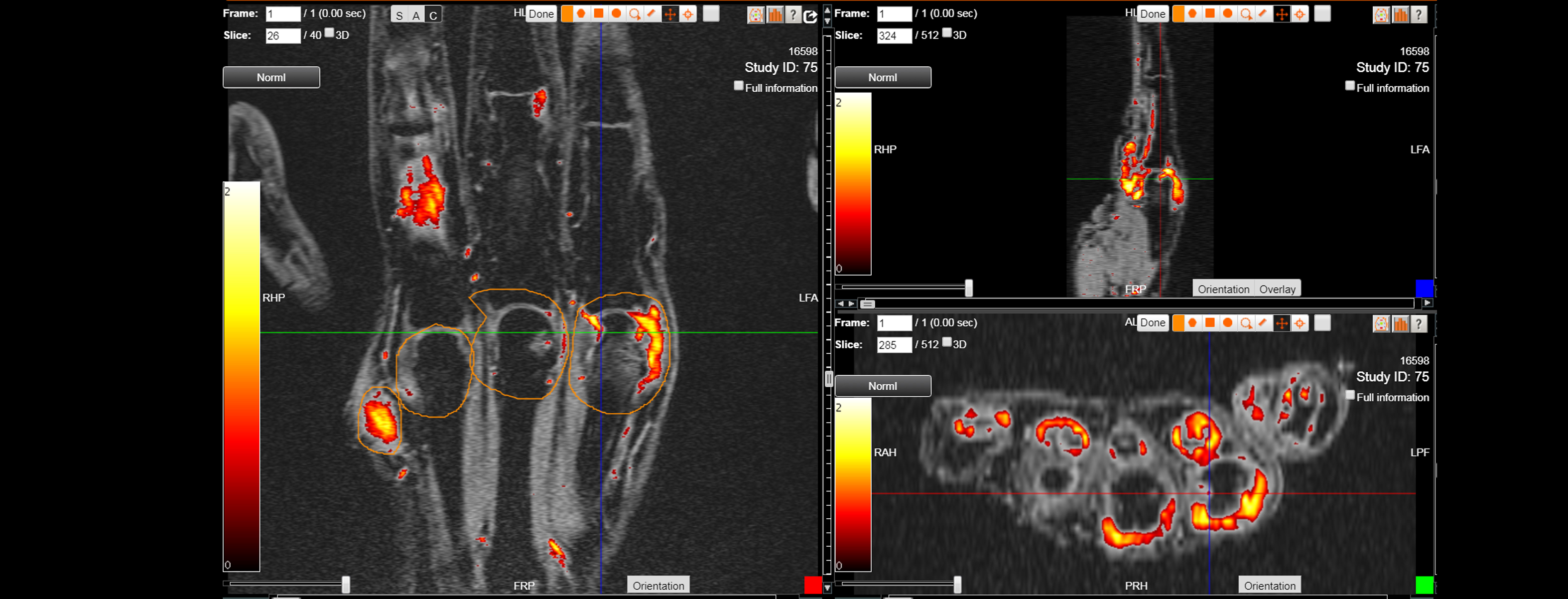
In monitoring RA, different clinical scores (i.e., DAS28) reflect the clinical disease activity. Clinical scores are limited and often despite of clinical remission, radiographic progression is possible, and the erosive process predicts the outcome of the disease. Objective imaging modalities are necessary to detect the destructive process as early as possible.
IAG’s team has extensive expertise and track record in supporting the development of RA’s blockbusters and helping to take these drugs from early phase all the way to phase 3 and approval. Our expertise is in use of
- X-ray
- MRI
- Dynamic Contrast Enhanced MRI
- CT
- PET (FDG and other tracers)
IAG’s team plays an active role in the scientific community and have led the development of novel scoring systems for early assessment of synovial inflammatory changes, quantification of inflammation (bone marrow oedema and synovial) and quantitative assessment of erosive changes.
About IAG, Image Analysis Group
IAG is a unique partner to life sciences companies developing new treatment and driving the hope of the up-coming precision medicine. IAG leverages expertise in medical imaging and the power of DYNAMIKA™, our proprietary cloud-based platform, to de-risk clinical development and deliver lifesaving therapies into the hands of patients much sooner. IAG provides early drug efficacy assessments, smart patient recruitment and predictive analysis of advanced treatment manifestations, thus lowering investment risk and accelerating study outcomes.
Acting as imaging Contract Research Organization, IAG’s experts also recognize the significance of a comprehensive approach to asset development. They actively engage in co-development projects with both private and public sectors, demonstrating a commitment to cultivating collaboration and advancing healthcare solutions.
Contact our expert team: imaging.experts@ia-grp.com
Experience: Scoring Systems
- Eligibility and Safety Assessments
- RA MRI Score (RAMRIS)
- Quantitative Inflammation (DEMRIQ)
- Radiographic Progression (Xray or CT)
- Erosive Volume
- US-Doppler Quantification
Experience: Imaging
- MRI
- DCE-MRI
- CT
- X-ray
- PET, PET-CT
- Ultrasound, US-Doppler
- MARs Imaging
Publications
Since 2007, over 2000 articles were published to cover scientific discoveries, technology break-throughs and special cases. We list here some critically important papers and abstracts.
Testimonials
Combining our technologies and business advisory services with promising life science companies has yielded spectacular results over the past five years. As a trusted partner to many biotech and pharma companies, IAG’s team is proud to share your words and quotes.

