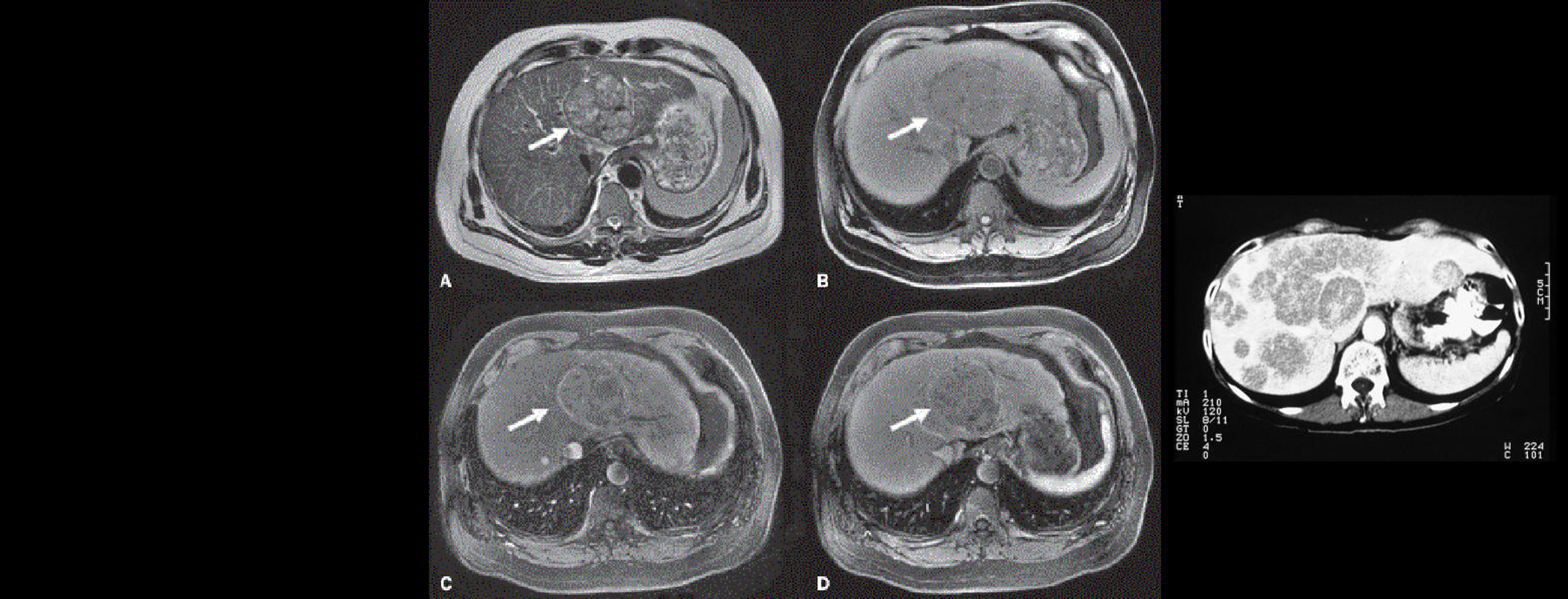

LIVER CANCER
Liver imaging in patients with a history of known or suspected malignancy is important because the liver is a common site of metastatic spread, especially tumours from the colon, lung, pancreas and stomach, and in patients with chronic liver disease who are at risk for developing hepatocellular carcinoma.
The goal of liver imaging in oncologic patients includes liver tumour detection and characterisation.
Liver biopsy is a standard procedure for diagnosing and staging non-alcoholic fatty-liver disease. However, biopsy has a number of disadvantages, including sampling error, intra- and inter-rater variability and poor patient acceptance due to potential complications. Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide.
Quantitative imaging is a promising approach for evaluating normal biological or pathogenic processes, as well as response to treatment and intervention without the need for invasive procedures. The field of hepatic MRI is relatively new compared with other organ systems as the complexity of hepatic imaging, including the dual blood supply of the portal vein and hepatic vessels, makes tracer imaging particularly challenging.
HCC commonly develops in the setting of concomitant or previous cirrhotic nodules or hepatic fibrosis, which can complicate classification of suspicious lesions using conventional imaging techniques alone. Depending on the imaging technique used (MRI, CT, US), various quantitative biomarkers can be extracted.
Proton Density Fat Fraction (PDFF)
Chemical-shift imaging is used to separate the liver signal into its fat and water parts. PDFF is the fraction of MRI-visible protons attributable to fat divided by all protons in the liver attributable to fat and water. This technique acquires GREs at appropriately spaced echo times. To increase examination accuracy, a low flip angle is used for minimizing T1 bias, along with multiple echoes for correcting T2* effects.
A standard MRI scanner of major manufactures will allow to reproducibly image patients and interpret images. The diagnostic accuracy of MRI-PDFF was validated by several studies, including Idilman et al and Bannas et al, which showed that MRI-based PDFF assessments closely correlated with histology acquired from liver biopsy.
MR Spectroscopy
MR Spectroscopy, similarly to liver biopsy, is collected from a single region positioned in the liver parenchyma and directly measures the differences in water and fat peaks on a resonance frequency domain. Clearly, the quantification is done only within a particular region which limits the comprehensive understanding of the liver disease. Currently, MRS is not available on all MR scanners, which confines its routine adoption for clinical trials and clinical practice.
MR Electrography
MR elastography uses a modified phase-contrast pulse sequence to visualize rapidly propagating mechanical shear waves. It is available in MRI scanners by major manufactures and can be acquired simultaneously with other MRI sequences. The best known application of MRE is in quantification of hepatic fibrosis.
Dynamic Contract Enhanced MRI
Currently, the use of DCE-MRI in both primary hepatic tumors and metastatic lesions are being investigated, as well as non-oncologic disease processes including hepatocellular fibrosis, cirrhosis, acute liver failure, and post-liver transplant rejection. DCE-MRI and tracer parameters can help differentiate regenerative, benign nodules from more concerning lesions. Sahani et al. reported models that differentiated blood flow and blood volume found differences in moderately or poorly differentiated HCCs. General concepts of differing microvascular structure and selection of appropriate anti-angiogenic therapies are important to maximize treatment response. DCE-MRI may assist in assessment of tumor response even before RECIST criteria can apply; early changes during therapy as demonstrated by perfusion parameters are not often seen on morphologic methods of imaging. Our upcoming publication will detail this further.
Our experience in liver imaging and quantitative biomarkers suggests that while there are multiple challenges in implementing MRI-PDFF, MRS, MRE, DCE-MRI in clinical trials, these techniques are reliable and noninvasive tools to assess hepatic steatosis, fibrosis and microvascular structure.In lights of sophisticated therapeutic developments and high treatment standards, we anticipate that in the next few years multiparametric MR imaging will become more of a standard, enabling better diagnosis and faster assessments of the treatment response.
References
- Sommer, W. H. et al. Contrast agents as a biological marker in magnetic resonance imaging of the liver: conventional and new approaches. Abdom. Imaging 37, 164–79 (2012).Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer45, 228–247. (2009)
- Yang, X. & Knopp, M. V. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol2011, 732848 (2011).
- Li, S. P. & Padhani, A. R. Tumor response assessments with diffusion and perfusion MRI. J. Magn. Reson. Imaging35, 745–63 (2012).
- Sourbron, S. P. & Buckley, D. L. Classic models for dynamic contrast-enhanced MRI. NMR Biomed.26(8), 1004–27 (2013).
- Hylton, N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J. Clin. Oncol.24, 3293–8 (2006).
- Ferl, G. Z. & Port, R. E. Quantification of antiangiogenic and antivascular drug activity by kinetic analysis of DCE-MRI data. Clin. Pharmacol. Ther.92, 118–24 (2012).
- Knopp, M., Giesel, F., Marcos, H., von Tengg-Kobligk, H. & Choyke, P. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Resnon Imaging12, 301–8. (2001).
- Padhani, A. & Husband, J. Dynamic contrast-enhanced MRI studies in oncology with an emphasis on quantification, validation and human studies. Clin Radiol56, 607–20 (2001).
- Padhani, A. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging16, 407–22 (2002).
- Tofts, P. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging7, 91–101 (1997).
- Barral, J. et al. A robust methodology for in vivo T1 mapping. Magn Reson Med64, 1057–67 (2010).
- DCE MRI Technical Committee. DCE MRI Quantification Profile, Quantitative Imaging Biomarkers Alliance. Version 1.0. Publicly Reviewed Version. Available from: RSNA.ORG/QIBA
- O’Connor, J., Jackson, A., Parker, G., Roberts, C. & Jayson, G. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol9, 167–77 (2012).
- Boesen, M. et al. Automatic Computer Aided Quantification Of Synovitis In Rheumatoid Arthritis Using Dynamic MRI And The Impact Of Movement Correction On Signal To Noise Ratio (SNR) And Region Of Interest (ROI) Analysis [abstract]. Arthritis Rheum60, 773 (2009).
- Kubassova, O. et al. in Med. Image Comput. Comput. Interv. – MICCAI. Lect. Notes Comput. Sci. Vol. 4792 261–269 (2007).
Experience: Scoring Systems
- LI-RADS
- RECIST1.1
- iRECIST
Experience: Imaging
- US
- CT
- MRI
- PET
Publications
Since 2007, over 2000 articles were published to cover scientific discoveries, technology break-throughs and special cases. We list here some critically important papers and abstracts.
Testimonials
Combining our technologies and business advisory services with promising life science companies has yielded spectacular results over the past five years. As a trusted partner to many biotech and pharma companies, IAG’s team is proud to share your words and quotes.

