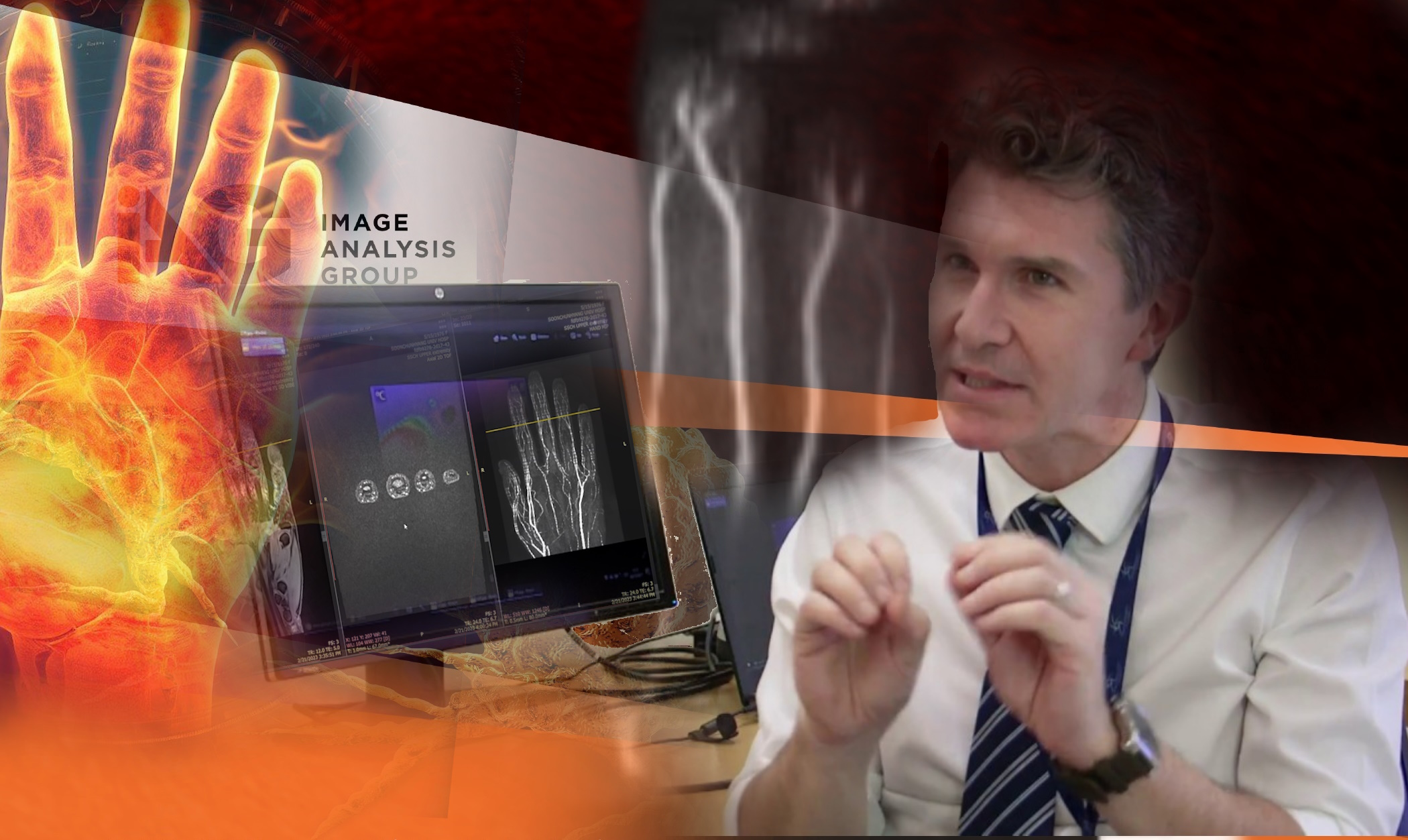
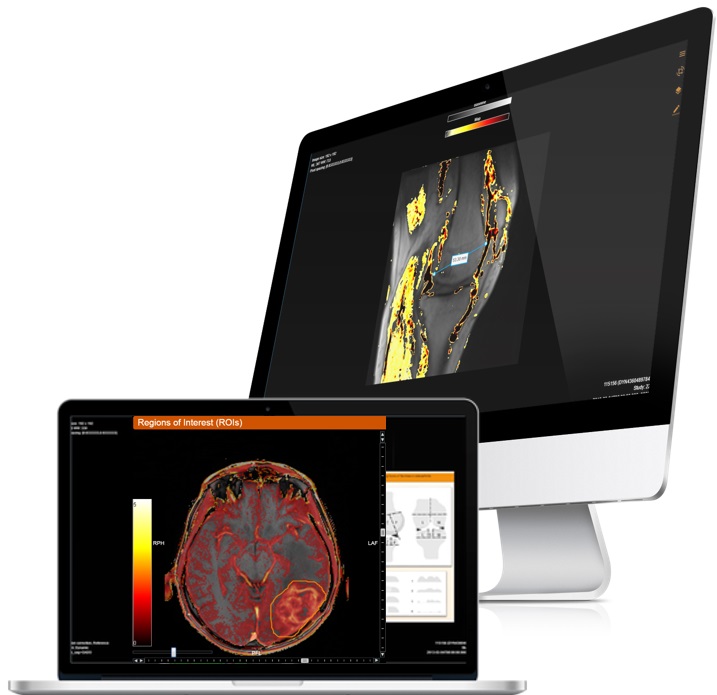
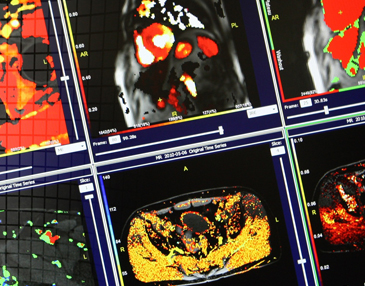
DYNAMIKA: Simplifying Central Imaging Review
DYNAMIKA is IAG’s enterprise-scale, cloud-based platform for imaging processing and data management, designed for multi-centre trial settings. The comprehensive software system enables:
- Central imaging quality control
- Streamlined trial progress
- Central reads
- Integration with AI tools for faster read-outs and decision support
Key features include:
- User access control
- Site data uploading
- Quality control
- Data preparation for reading
- Range of industry-standard reading protocols
- Data export
- Study progress reporting
DYNAMIKA has powered numerous global clinical studies, from Phase I-IV, managing most modalities in key therapeutic areas. Over the past decade, we engaged with sites around the world, processing complex imaging data from thousands of patients.