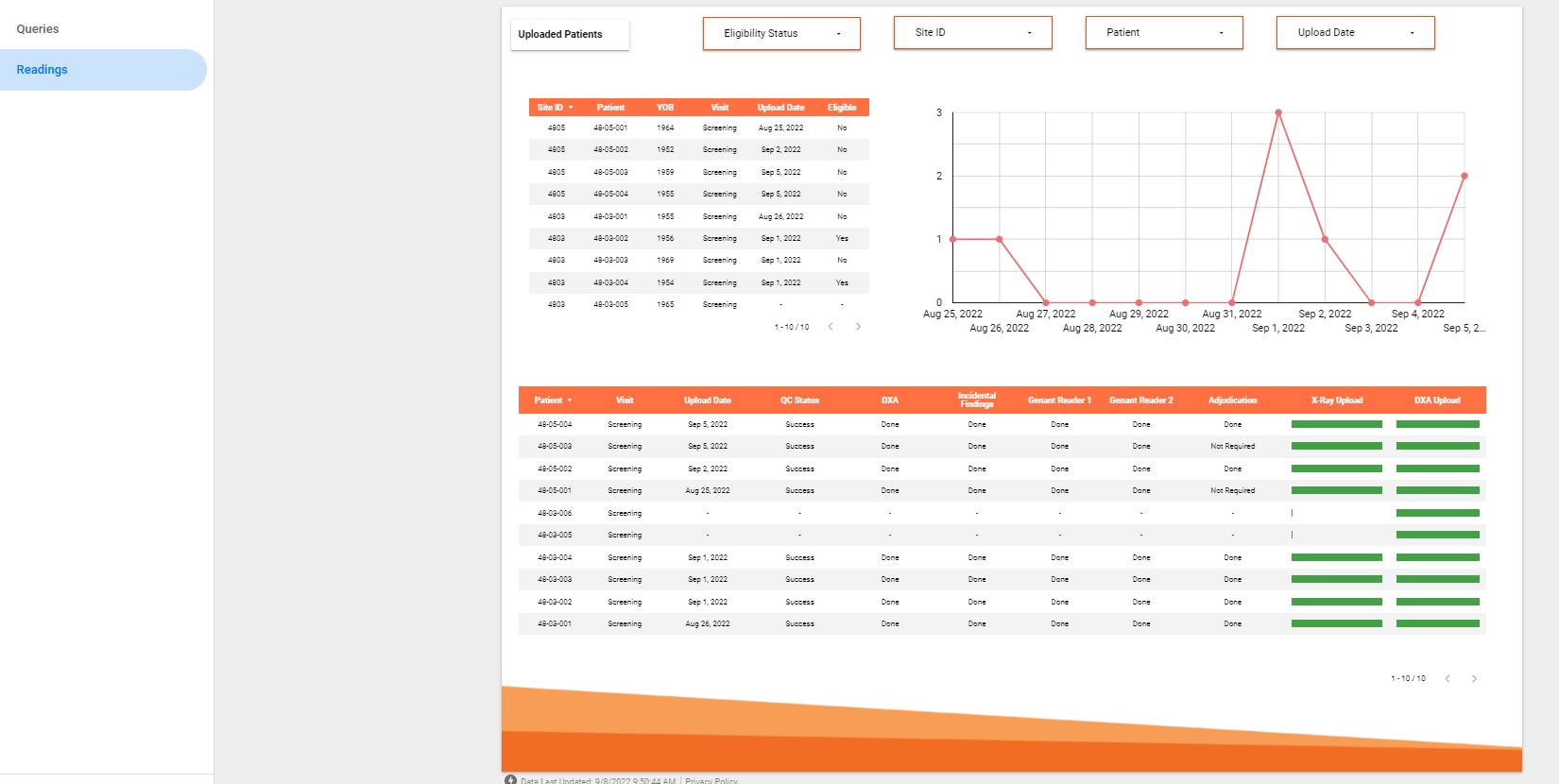
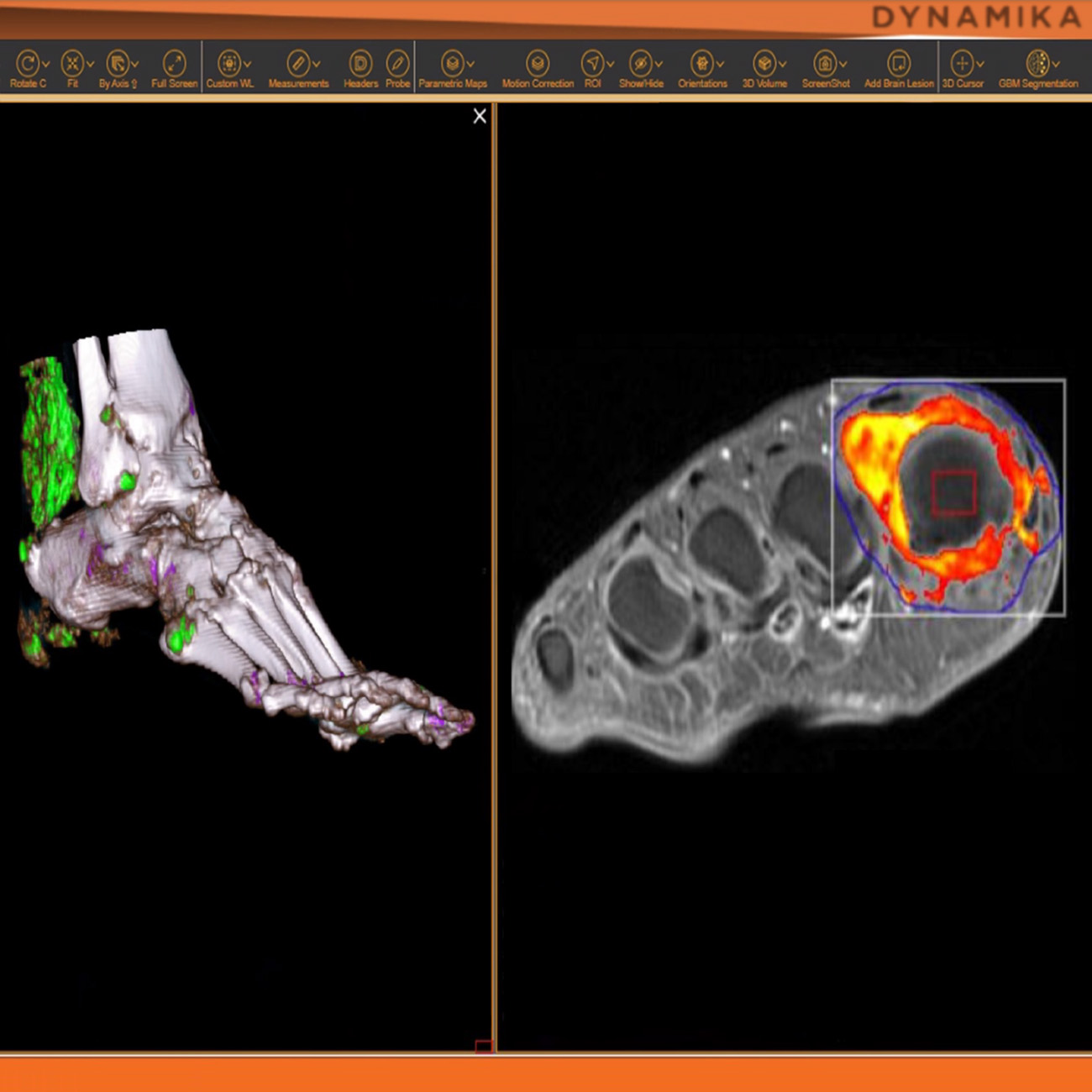
IAG brings many years of experience in advising on and delivering imaging critical clinical trials, where advanced imaging-based biomarkers provide critical evidence of the novel compound safety and efficacy.
Our platform DYNAMIKA is developed under ISO13485 and compliant through the use of Quality Management System (QMS) with Standard Operating Procedures. IAG is audited by clients on regular basis. The QMS is audited on annual basis by British Standards Institute (BSI) and bi-annually by internal auditors, prompting continuous improvement of our processes and procedures.