
Turning Phase III Failure into Success: A Case Study in Innovative Imaging Analysis for Rare Disease Trials
Turning Phase III Failure into Success: A Case Study in Innovative Imaging Analysis for Rare Disease Trials
In the challenging landscape of rare disease drug development, a global pharmaceutical company faced a significant setback when their Phase III trial failed to meet primary and secondary endpoints. This case study demonstrates how innovative imaging analysis strategies can salvage seemingly failed trials and provide crucial insights for biotech leadership.
The Challenge
Phase III trial failure in a rare disease affecting growing children
Highly subjective clinical tests not reflecting true treatment efficacy
Nearly 250 sites with low patient volume, leading to image acquisition and quality issues
25% of images classified as ‘poor quality’, with remaining data being borderline to good quality
IAG’s Innovative Approach
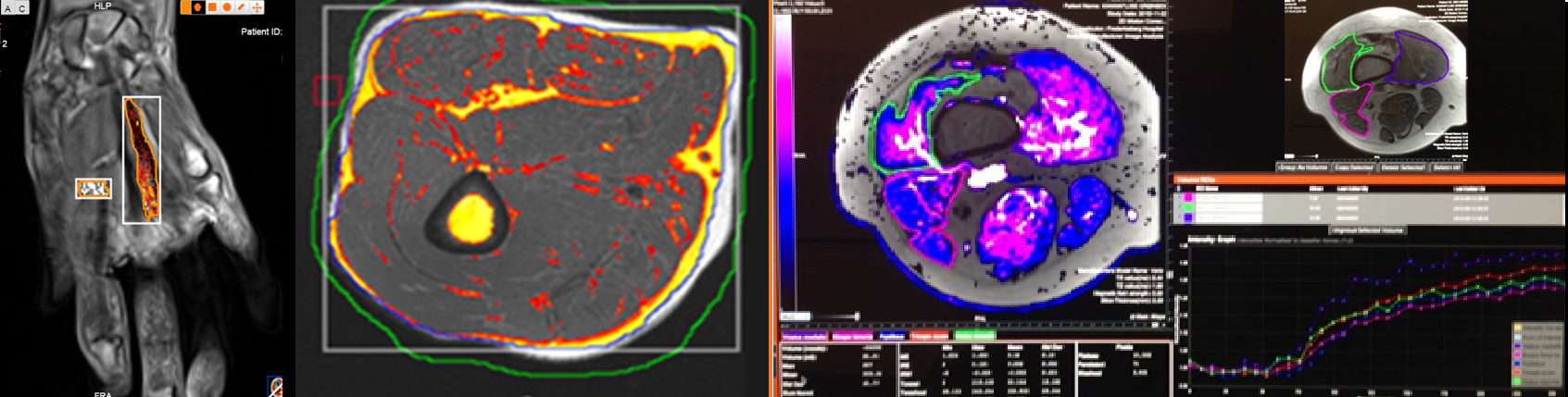
IAG’s approach in this case study centered on comprehensive data re-analysis and advanced imaging techniques. After the initial Phase III trial failure, IAG conducted a thorough quality assessment of all existing imaging data, classifying images based on their quality. This process revealed that nearly 25% of images were of poor quality, with the remaining data ranging from borderline to good quality. To address this issue, IAG designed a specific methodology for quantifying treatment efficacy that could account for varying image quality levels.
The company employed advanced imaging analysis techniques, utilizing computer-aided quantification to produce continuous data rather than relying solely on traditional radiological assessments. This approach allowed for the capture of subtle treatment-induced changes that might have been missed by conventional methods, particularly important in this rare disease affecting growing children where standard clinical tests were highly subjective and did not account for individual growth and development.
Furthermore, IAG implemented innovative statistical analysis techniques, including weighting results based on image quality and acceptability. They employed a blinded reading process for the operational team and readers to enhance objectivity. This comprehensive approach enabled IAG to differentiate treatment groups and achieve statistically significant results with fewer data points and at earlier timepoints, potentially salvaging a trial that had initially appeared to fail in meeting its endpoints. By leveraging these advanced methodologies, IAG demonstrated the value of expert imaging analysis in overcoming challenges in rare disease clinical trials.
Key Outcomes
The innovative approach yielded significant results within months:
Differentiation between treatment groups became possible
Statistically significant results achieved with fewer data points
Earlier timepoint identification for treatment efficacy
Increased reproducibility of scoring due to automation
Implications for Biotech Leadership
Data Quality Management: Implement real-time quality control measures to ensure usable data, especially in multi-center trials with low patient volume.
Innovative Analysis Techniques: Explore advanced quantification methods that can extract more sensitive information from imaging data.
Adaptive Trial Design: Be prepared to re-evaluate and adapt trial methodologies when faced with initial negative results.
Technology Integration: Leverage cloud-based infrastructure and automation to enhance data management and analysis efficiency.
Strategic Partnerships: Collaborate with imaging experts early in the trial process to optimize data collection and analysis strategies.
This case study demonstrates how innovative approaches to imaging analysis can revitalize seemingly failed trials in rare disease research. By implementing advanced technologies and methodologies, biotech companies can extract valuable insights from existing data, potentially saving years of research and millions in development costs.
