
Clinical experience with the AKT1 inhibitor miransertib in two children with PIK3CA-related overgrowth syndrome
The clinical and radiographic symptoms of Proteus syndrome are highly variable, as are its orthopaedic manifestations.
IAG, Image Analysis Group worked alongside Arqule (acq. by Merck) and outstanding academics led by Dr. Irvine to support the development of novel imaging-based system for assessment of the drug efficacy.
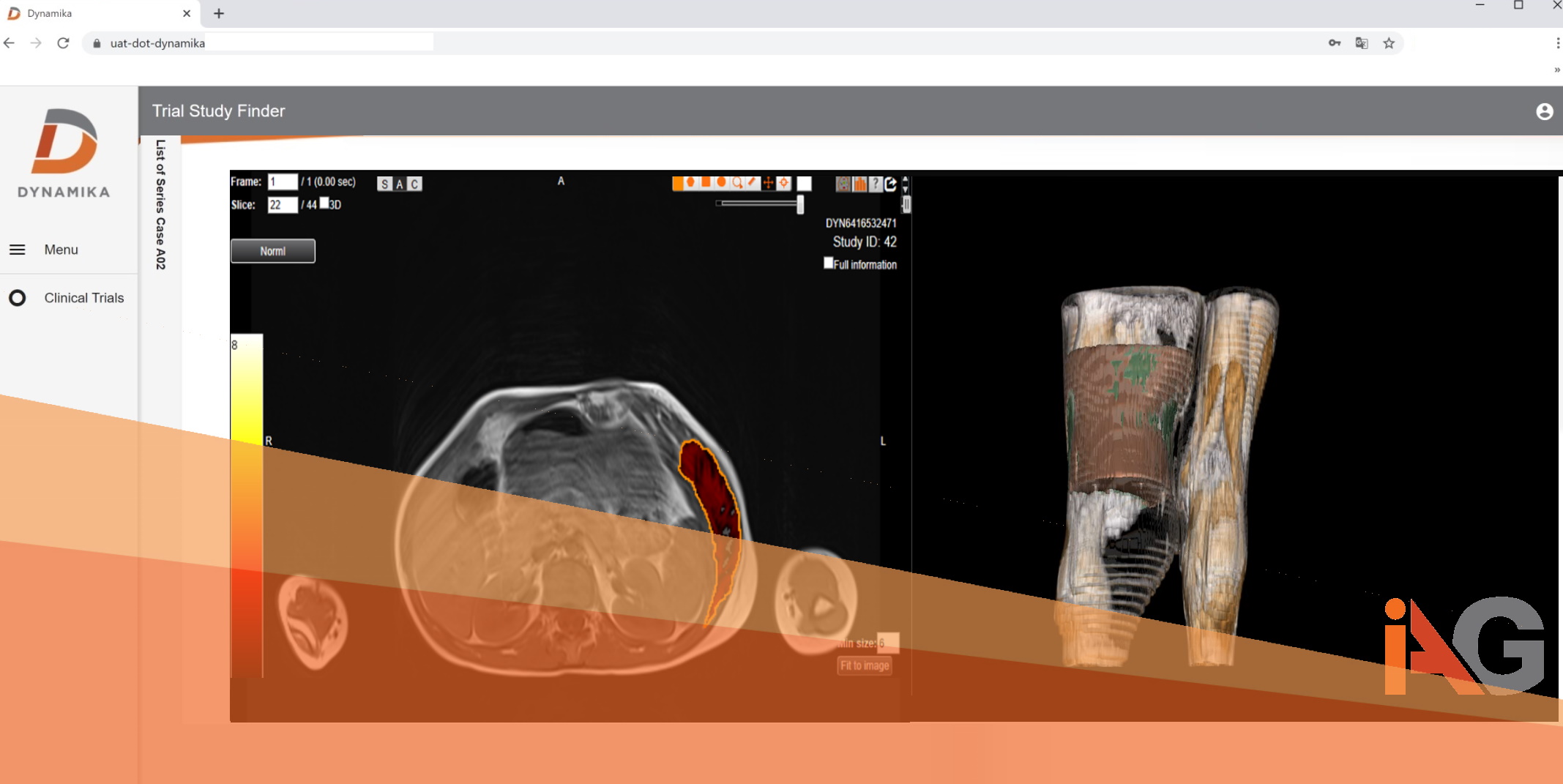
Its implementation in our platform DYNAMIKA and subsequent application in the trial led to clearer definitions of the radiographic changes in these patients and ability to derive quantitative measurable outcomes in this small trial.
This publication reports the first paediatric case series of the use of Miransertib in two children with PROS (PIK3CA-related overgrowth spectrum).
Objective clinical response was observed in patient one, and improvement in key qualitative outcomes was reported in patient two. This case series highlights the potential therapeutic utility of Miransertib in selected paediatric patients with severe PROS, and further demonstrates the potential for re-purposing targeted therapies for the treatment of rare diseases.
An open label, Phase 1/2 study of Miransertib in children with PROS and PS is underway to more accurately assess the efficacy of Miransertib in the treatment of PROS disorder (NCT03094832).
IAG contributed to this investigation led by Arqule (now acquired by Merck) by designing and deploying a imaging based scoring system and conducting the review of all patients in DYNAMIKA. The scoring systems allowed to assess volumetric and morphological changes in series of MRI analysis, and revealed up to 15% reduction in calculated volumes of fatty overgrowth between the treatment commencement and the last follow-up.
Title: ‘Clinical experience with the AKT1 inhibitor Miransertib in two children with PIK3CA-related overgrowth syndrome’
Journal: Orphanet Journal of Rare Diseases, February 2021
Authors: Karina Forde, Nicoletta Resta, Carlotta Ranieri, David Rea, Olga Kubassova, Mark Hinton, Katrina A. Andrews, Robert Semple, Alan D. Irvine & Veronika Dvorakova
Access Online: https://ojrd.biomedcentral.com/articles/10.1186/s13023-021-01745-0
About Image Analysis Group (IAG)
IAG, Image Analysis Group is a unique partner to life sciences companies. IAG leverages expertise in medical imaging and the power of Dynamika™ – our proprietary cloud-based platform, to de-risk clinical development and deliver lifesaving therapies into the hands of patients much sooner. IAG provides early drug efficacy assessments, smart patient recruitment and predictive analysis of advanced treatment manifestations, thus lowering investment risk and accelerating study outcomes. IAG bio-partnering takes a broader view on asset development bringing R&D solutions, operational breadth, radiological expertise via risk-sharing financing and partnering models.
Learn more: wp1.ia-grp.com
Reach out: imaging.experts@ia-grp.com
Follow the Company: Linkedin
