
Novel quantitative digital image analysis methodology for assessment of inflammatory changes in MRI data in a post-hoc analysis of data acquired from a phase IIb study of baricitinib in patients with active rheumatoid arthritis
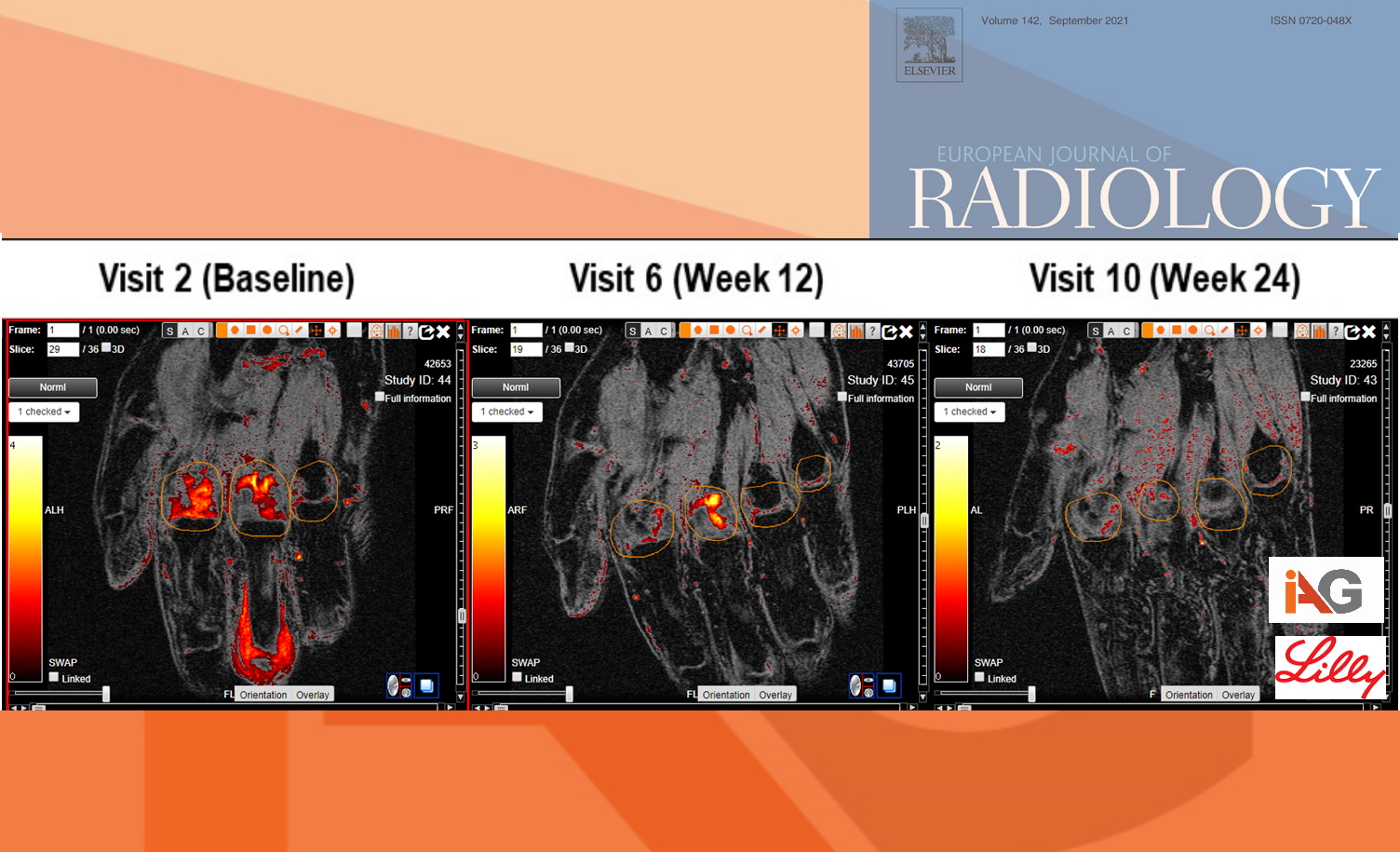
Image Analysis Group, the imaging expert company supported central imaging data review from the Phase II trial of Baricitinib, an oral JAK inhibitor (NCT01185353) conducted by Eli Lilly, an American pharmaceutical company. The teams collaborated to further assess inflammatory changes in MRI data of RA patients using IAG’s proprietary quantitative methodology that allowed to extract a continuous volume of inflammation and to assess the severity of inflammation. These quantitative imaging biomarkers are complimentary to radiological score for RA (RAMRIS) and can be extracted from the same MR images. These AI-driven quantitative imaging techniques bring deeper understanding of inflammatory changes at specific joints and assessment of treatment impact at earlier timepoint. In this case, the changes were visible as early as week 12. Furthermore, this article explores the use of MRI in RA trials and discusses the optimal set-up at the imaging sites and stringent quality control that are paramount to acquiring imaging data of high quality.
Read more: Novel quantitative digital image analysis methodology for assessment of inflammatory changes in MRI data in a post-hoc analysis of data acquired from a phase IIb study of baricitinib in patients with active rheumatoid arthritis, Boest et al., https://pubmed.ncbi.nlm.nih.gov/34412009/
A groundbreaking study published in 2021 introduces a novel quantitative digital image analysis methodology that promises to transform rheumatoid arthritis (RA) clinical trials. This innovative approach, developed by Image Analysis Group (IAG) in collaboration with Eli Lilly, offers pharmaceutical companies a powerful new tool to accelerate drug development and improve patient outcomes.
Key Advantages for Drug Developers:
-
Earlier Efficacy Assessment: Detect treatment effects as early as week 12, potentially shortening trial durations and reducing costs.
-
Enhanced Sensitivity: AI-driven quantitative imaging techniques provide deeper insights into inflammatory changes at specific joints, offering a more nuanced understanding of treatment impact.
-
Complementary to Existing Measures: The new methodology works alongside the established RAMRIS score, extracting additional value from the same MRI data.
-
Highly Reproducible: With intraclass correlation coefficients (ICC) ranging from 93% to 99%, the method ensures consistent and reliable results across trials.
-
Optimized Trial Design: Insights on imaging site setup and quality control protocols help improve overall trial data quality.
Study Highlights:
-
Over 160 patients treated with Baricitinib and imaged at baseline, week 12, and week 24
-
Utilization of Dynamic Contrast Enhanced (DCE) MRI for advanced pharmacokinetic modeling
-
Extraction of continuous volume of inflammatory changes using IAG’s proprietary AI methodology
-
Demonstrated ability to differentiate treatment groups with fewer patients and at earlier timepoints
This innovative approach, validated in a phase IIb study of Baricitinib (NCT01185353), offers pharmaceutical companies a powerful tool to accelerate RA drug development, potentially reducing time-to-market and development costs while improving the likelihood of trial success.
By partnering with IAG and leveraging their Dynamika™ platform, drug developers can access cutting-edge imaging expertise to de-risk clinical development and bring life-saving therapies to patients sooner.
