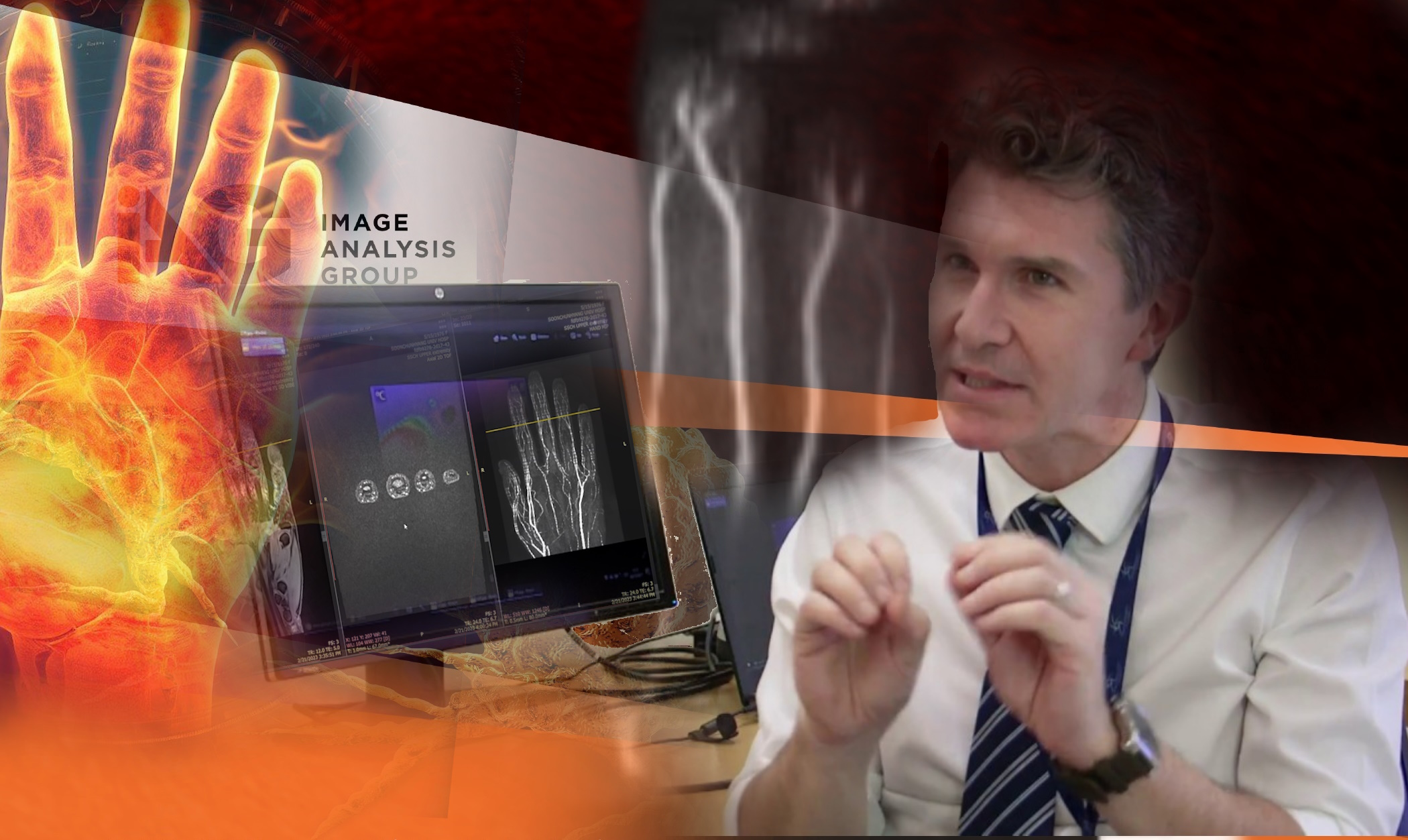

The Lancet Rheumatology Publishes the Data from Prospective Study on DAVIX, the Surrogate Outcome Measure for Patients with Systemic Sclerosis
We are delighted to announce the publication of a research paper titled “MRI Digital Artery Volume Index (DAVIX) as a surrogate outcome measure of digital ulcer disease in patients with systemic sclerosis: a prospective cohort study” in the Lancet Rheumatology (impact factor 25.4).
This study is a result of successful collaboration between the dedicated team of scientists, physicians and patients.
‘It not only offers hope for systemic sclerosis patients through the introduction of DAVIX as a surrogate outcome measure but also aligns with the goals of early treatment response assessment in scleroderma drug development,’ commented Prof. del Galdo, PI on the study.
To our knowledge, this is the first study to use a semi-automated, MRI based technique to quantitatively assess digital artery disease in patients with systemic sclerosis and to explore its clinical value by correlating the resulting index with clinical parameters and patient-reported outcomes.
MRI Digital Artery Volume Index (DAVIX) as a surrogate outcome measure of digital ulcer disease in patients with systemic sclerosis: a prospective cohort study, M. Hughes, S. Di Donato, K. Gjeloshi, G. Abignano, F. Danzo, G. Lettieri, E. De Lorenzis, D. Bertham, P. O’Connor, O. Kubassova, J. Dehmeshki, F. Del Galdo, Volume 5, Issue 10, E569-E570, Oct. 2023
DAVIX is currently being used in two multicentre randomised controlled trials (NCT05559580 and CM101 in systemic sclerosis).
Both studies will investigate the feasibility of adopting DAVIX as a surrogate imaging outcome measure of peripheral vascular disease and consider the effects of using different MRI magnets and recruiting from different geographical locations, among other endpoints.
‘DAVIX’s ability to non-invasively predict future digital ulcers and worsening of patient-reported outcomes could aid patient enrichment and stratification in clinical trials’, said Dr. Kubassova, CEO of IAG, Image Analysis Group.
This publication is a testament to the work done by teams at the University of Leeds, Image Analysis Group and collaborators.
About Image Analysis Group (IAG)
IAG, Image Analysis Group is a unique partner to life sciences companies. IAG leverages expertise in medical imaging and the power of Dynamika™ – our proprietary cloud-based platform, to de-risk clinical development and deliver lifesaving therapies into the hands of patients much sooner. IAG provides early drug efficacy assessments, smart patient recruitment and predictive analysis of advanced treatment manifestations, thus lowering investment risk and accelerating study outcomes. IAG bio-partnering takes a broader view on asset development bringing R&D solutions, operational breadth, radiological expertise via risk-sharing financing and partnering models.
Learn more: wp1.ia-grp.com
Reach out: imaging.experts@ia-grp.com
Follow the Company: Linkedin
