
LUNG CANCER
Lung cancer is usually diagnosed in advanced stages with majority of patients already presenting metastatic disease. Only 20 % of NSCLC patients have early stage disease at the time of diagnosis, thus being potentially resectable. The current gold standard is lobectomy with hilar and mediastinal lymph-node sampling or dissection. Curative stereotactic body radiotherapy (SABR) should be offered to patients with stage I NSCLC who have clinical comorbidities or are at very high surgery-related risk, and those who refuse to undergo surgical procedure. Post-operative platinum-based chemotherapy is recommended for all patients with stage II and III surgically resected disease. For patients with locally advanced stage NSCLS, there are different recommended options, including
- Surgery followed by adjuvant chemotherapy
- Neoadjuvant chemotherapy followed by surgery
- Neoadjuvant chemo-radiation followed by surgery
Eligibility for pre-operative or post-operative platinum-doublets with or without radiotherapy should be evaluated in the context of an experienced multidisciplinary team.
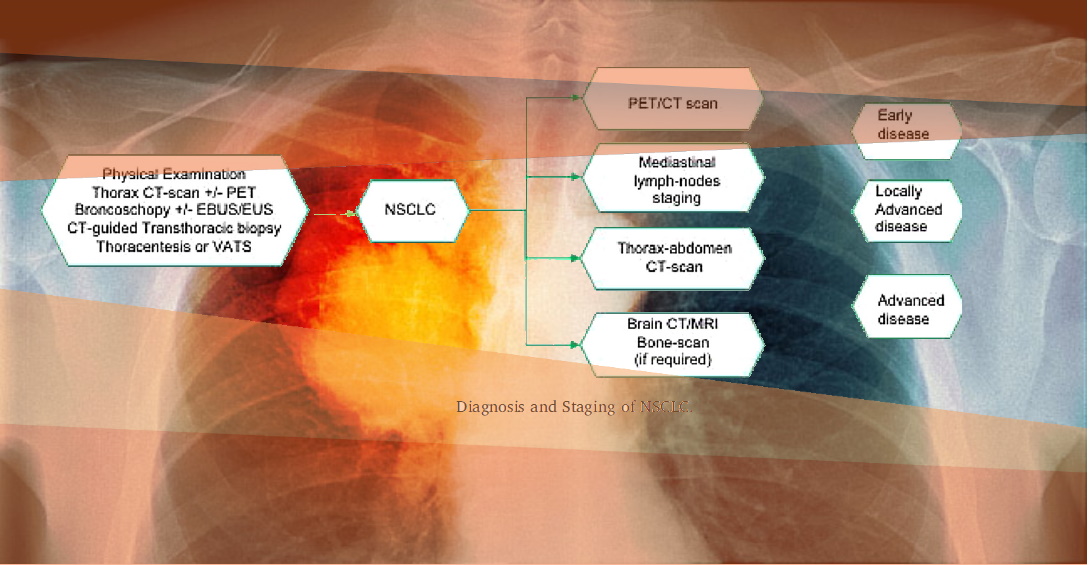
The diagnostic evaluation initially focuses on careful physical examination and patient’s history, to identify new symptoms or a significant change in the common respiratory symptoms.
In clinical trials, all patients with suspected lung cancer will undergo a non-invasive chest imaging, including X-rays, CT-scan, and if needed positron emission tomography (PET) with fluorodeoxyglucose (FDG).
Conventional contrast-enhanced chest CT-scan is considered the best exam to detect lung cancer, as it provides detailed information on anatomic location, margins, invasion of surrounding structures or chest wall, and mediastinal lymph nodes involvement.
Use of imaging presents a great opportunity for selecting the right patients and enriching the trial.
IAG offers multiple imaging technique solutions, which are utilized in clinical trials both, while scanning patients for inclusion in the clinical studies, and while assessing the efficacy of ongoing treatments.
- CT Imaging – Computed Tomography (CT) scan of the chest area is the keystone of lung cancer imaging based on which further management options are decided.
- SPECT imaging – A single-photon emission computed tomography (SPECT) renders tomographic imaging, which could be a more accurate method for regional valuation by diagnosing radioactivity in all pulmonary lobes, avoiding spatial overlapping.
- ctDNA testing – Without the risks inherent to biopsy, ctDNA can be attained over time permitting for some serial assessments. Numerous clinical studies have furthermore suggested that ctDNA can be used to identify the occurrence of minimal residual disease (MRD) post-surgical resection in various cancer types, especially lung cancer.
- EGFR/ALK/ROS/BRAF testing – For targeted therapies, with Mechanism of actions targeting multiple mutations in non-small-cell lung cancer (NSCLC) patients, EGFR/ALK/ROS/BRAF testing imaging techniques are useful to assess the patients for eligibility in the trial as well to assess the treatment efficacy.
- MRI and ECG – MRI and ECG scans are typically utilized to assess the organ functions, before including patients in the Lung cancer clinical trials.
At IAG, we design and implement protocols for thoracic CT images, assess the need for breath hold and use of intravenous contrast. We support image reconstruction, quality control and reading.
To optimise nodule detection, normally all baseline CTs are read by experienced thoracic radiologists; sometimes located in the local trial centres or at a central site. All discrepancies and adjudication is done by IAG’s imaging specialists. It is common to ask the readers to identify and record all lung nodules greater than a certain size and diameter. We deploy volumetric measures whenever possible. Our measurements include the volume and maximum intensity projections (MIPs) to aid the detection.
MRI exams are done in addition to CT when we are trying to assess the drug efficacy in a specific way, such as 1) resolution of the tumour invasion into the chest wall and the mediastinal structures (pancoast tumour); 2) impact on the solid and vascular hilar masses; 3) impact on the diaphragmatic abnormalities or when following-up mediastinal lymphoma.
MR exams are susceptible to motion artefacts such as breathing and require specific software to process. Recently, new applications, such as whole-body MR (WBMR) imaging are being deployed to assess metastatic disease. Diffusion weighted imaging (DWI) is used to assess changes in the tumour cellularity and the integrity of the cellular membrane. The DWI sequence is made susceptible to the differences in water mobility. The motion of water molecules is more restricted in tissues with a high cellular density associated with numerous intact cell membranes (e.g. tumour tissue). This technique can be applied for tumour detection and tumour characterisation and for the monitoring of response to treatment.
PET/CT is a combined imaging technique: CT giving anatomical information and PET giving metabolic information to detect lesions initially not seen on CT and to assess more precise localisation of lesions, delineate them from their surrounding structures and provide more accurate characterisation of a lesion as benign or malignant.
Reach out to our expert team, as you are designing and planning your trial.
About IAG, Image Analysis Group
IAG is a unique partner to life sciences companies developing new treatment and driving the hope of the up-coming precision medicine. IAG leverages expertise in medical imaging and the power of DYNAMIKA™, our proprietary cloud-based platform, to de-risk clinical development and deliver lifesaving therapies into the hands of patients much sooner. IAG provides early drug efficacy assessments, smart patient recruitment and predictive analysis of advanced treatment manifestations, thus lowering investment risk and accelerating study outcomes.
Acting as imaging Contract Research Organization, IAG’s experts also recognize the significance of a comprehensive approach to asset development. They actively engage in co-development projects with both private and public sectors, demonstrating a commitment to cultivating collaboration and advancing healthcare solutions.
Contact our expert team: imaging.experts@ia-grp.com
Experience: Scoring Systems
- RECIST1.1
- iRECIST
Experience: Imaging
- CT
- PET/CT
- MRI
- DWI
- WBMRI
- PET
Publications
Since 2007, over 2000 articles were published to cover scientific discoveries, technology break-throughs and special cases. We list here some critically important papers and abstracts.
Testimonials
Combining our technologies and business advisory services with promising life science companies has yielded spectacular results over the past five years. As a trusted partner to many biotech and pharma companies, IAG’s team is proud to share your words and quotes.

