
RENAL CELL CARCINOMA (RCC)
Renal cell carcinoma (RCC) is the most common primary renal malignant neoplasm in adults. It accounts for approximately 90% of renal tumors and 2% of all adult malignancies. The preferred method of imaging renal cell carcinomas is dedicated renal computed tomography (CT). High resolution, reproducibility, reasonable preparation and acquisition time, and acceptable cost allow CT to remain as the primary choice for radiologic imaging.
MRI is an important alternative. The primary limitation of CT is the characterization of hypoattenuation in masses smaller than 8-10 mm, in which pseudo-enhancement may be a problem. In addition, spread to regional lymph nodes in the absence of lymph node enlargement can be missed.
If contrast material cannot be intravenously administered, CT is a poor choice for evaluating renal masses.
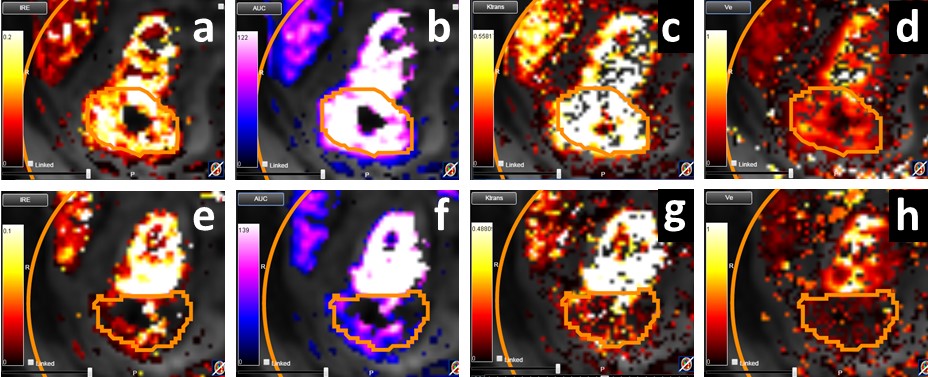
MRI is an important alternative to CT as it is much more sensitive. We have carried out the first reported multiparametric MRI (mpMRI) analysis of tumour diffusion and perfusion changes in response to Stereotactic ablative body radiotherapy (SABR) treatment of RCC.
Our latest work ‘Tumour response assessment on multi-parametric MRI after stereotactic ablative body radiotherapy for primary kidney cancer’ was presented as an oral presentation at ICCR 27-30 June 2017, London, UK.
In the recently completed phase IIb clinical trial in this context (FASTRACK, trial number U1111-1132-5574, https://clinicaltrials.gov/ct2/show/NCT01676428 ), dose delivered was dependent on tumour size with lesions ≤5 cm diameter receiving a single fraction of 26 Gy and larger lesions three fractions of 14 Gy prescribed to the 99% of the target volume. While it has been shown SABR of RCC can achieve high local control rates, variable degrees of morphological tumour shrinkage are typical, with residual masses being common place for sustained periods post-treatment.
Multi-parametric MRI (mpMRI) can be used to investigate radiation induced changes, and in this study, we conduct an exploratory analysis of diffusion and perfusion changes in RCC tumours after SABR using diffusion-weighted (DWI) and dynamic contrast enhanced (DCE) MRI.
Study objectives were to a) establish a methodology for mpMRI evaluation of RCC response to SABR and b) evaluate the potential utility of mpMRI as a biomarker of treatment response.
Results showed that ADC measures and IRE and AUC parametric maps show promise for indicating patient response after SABR. These parameters may provide novel early response biomarkers in a disease for which conventional CT based geometric RECIST response criteria are presently inadequate. Motion of the kidney during mpMRI acquisition and tumour contouring provided challenges to analysis, however we expect our work to implement 3D motion correction to account for kidney movement in DCE MRI will improve data quantification, particularly towards reproducibility of pharmacokinetic maps.

[1] S. Siva, et al., “A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma,” BJU Int., vol. 110, pp. 737–743, 2012.
[2] D. Pham, et al., “Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity.,” Int. J. Radiat. Oncol. Biol. Phys., vol. 90, no. 5, pp. 1061–8, 2014.
[3] E. A. Eisenhauer, et al., “New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1),” Eur. J. Cancer, vol. 45, no. 2, pp. 228–247, 2009.
[4] P. S. Tofts, et al., “Estimating kinetic parameters from DCE T1w MRI of a Diffusable Tracer: Standardized Quantities and Symbols,” J. Magn. Reson. imaging, vol. 10, pp. 223–232, 1999.
Experience: Scoring Systems
- RECIST 1.1
- irRC (Immune-Related Response Criteria)
- iRECIST
- mRECIST
- PET Response Criteria in Solid Tumors (PERCIST)
- PET-based Standard Uptake Value (SUV)
- Volumetric Assessment
- Dual input function renal perfusion assessment
Experience: Imaging
- CT
- DW-MRI
- DCE-MRI
- PET / CT
Publications
Since 2007, over 2000 articles were published to cover scientific discoveries, technology break-throughs and special cases. We list here some critically important papers and abstracts.
Testimonials
Combining our technologies and business advisory services with promising life science companies has yielded spectacular results over the past five years. As a trusted partner to many biotech and pharma companies, IAG’s team is proud to share your words and quotes.

