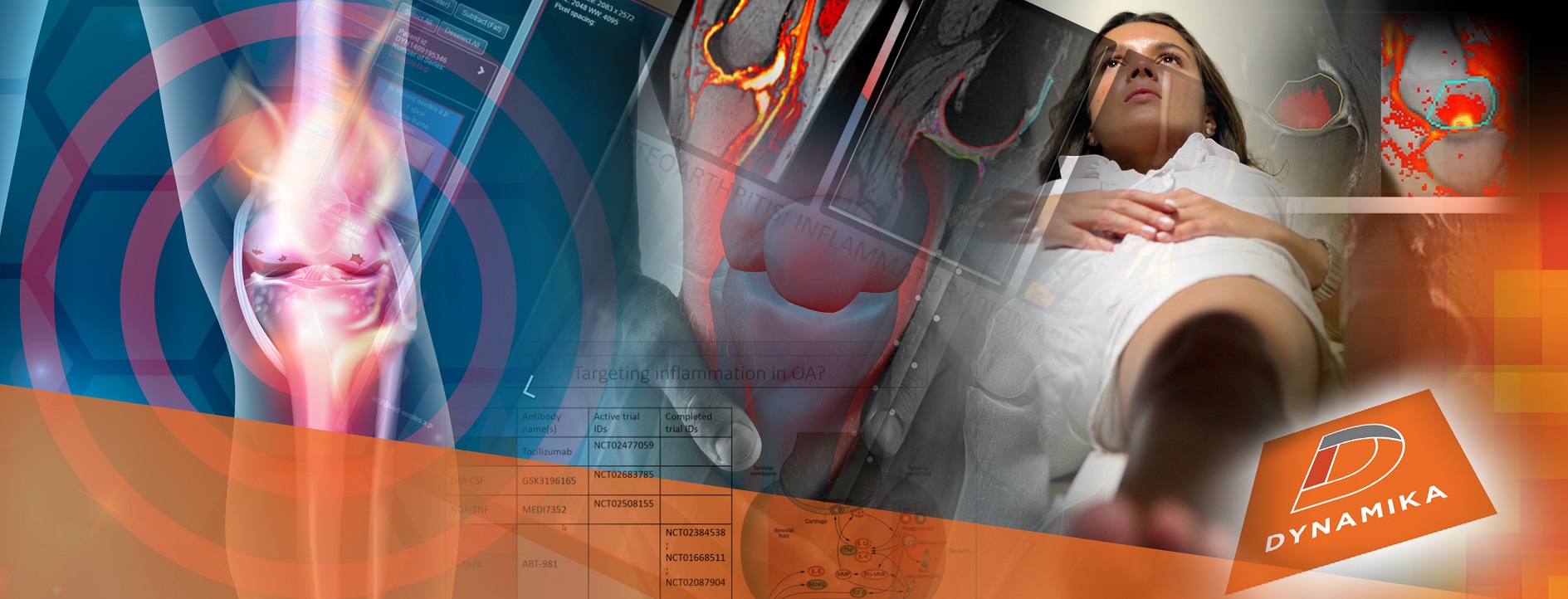

Introduction
The quest for effective cartilage repair has long been a challenge in the field of orthopedics and regenerative medicine. Cartilage, which cushions and supports our joints, is notorious for its limited self-healing capacity and was hard to visualise using x-ray-based techniques. However, thanks to advancements in medical imaging, particularly Magnetic Resonance Imaging (MRI), researchers and clinicians are making significant strides in understanding cartilage health and developing innovative treatments for cartilage repair.
MRI in Cartilage Assessment
One of the most compelling advantages of MRI in cartilage assessment is its non-invasive nature. Traditional diagnostic approaches, like arthroscopy, require surgical procedures that come with inherent risks such as infection, anesthesia complications, and extended recovery times. In contrast, owing to its unparalleled soft tissue contrast, MRI is the preferred non-invasive method for assessing the joint cartilage. It allows clinicians to evaluate the thickness, hydration, and integrity of cartilage layers without making a single incision. Patients undergoing MRI experience minimal discomfort and can often resume their regular activities immediately following the scan. This significantly lowers the threshold for early screening and regular monitoring.
Multi-Modal Imaging
MRI is far from a one-size-fits-all imaging technique, especially when it comes to assessing the complex, multi-layered structure of cartilage. Advanced MRI techniques, such as T1ρ (T1 rho) and T2 mapping, along with texture analysis, have significantly expanded our ability to scrutinize different properties of cartilage in unprecedented detail.
- T1ρ Mapping: This technique is particularly useful for assessing the proteoglycan content in cartilage. Proteoglycans are essential for the cartilage’s load-bearing function. A depletion in their concentration often signals early degenerative changes. T1ρ mapping provides quantitative data on proteoglycan density, aiding clinicians and researchers in determining the severity of cartilage damage.
- T2 Mapping: T2 relaxation time mapping is a technique that reflects the interaction between water and the extracellular matrix in tissues. This provides a visual representation of the collagen framework and the hydration level within the tissue. In MRI images captured with long repetition times, T2 effects primarily influence the visibility and contrast of the layers of hyaline cartilage. T2 mapping is a major MRI method for detecting early degenerative changes in cartilage. In the realm of cartilage repair, clinicians utilize quantitative T2 mapping to assess the zonal structure of the repair tissue, thus allowing for the monitoring of its maturation over time.
- Texture Analysis: Another growing area in advanced MRI techniques for cartilage assessment is texture analysis. This method involves computational evaluation of the spatial arrangement of pixel intensities in an image. It provides insights into patterns and variations within the cartilage structure. Texture features can help discern early alterations in cartilage health, enhancing the robustness of the multi-modal approach.
Early Detection
One of the most significant benefits of MRI technology is its capability for early detection of cartilage issues. Unlike other imaging methods, MRI can identify the initial stages of cartilage wear and tear with a high degree of accuracy. This is crucial for timely intervention, given cartilage’s limited ability to heal itself. Early diagnosis opens up a wider range of treatment options.
The importance of early detection goes beyond just immediate treatment; it also has long-term implications for preventing conditions like osteoarthritis. This debilitating disease, often caused by cartilage deterioration, can seriously impact a person’s quality of life. Utilizing MRI for early diagnosis can better manage or even slow the progression of such chronic conditions, offering patients a significantly improved outlook.
New Treatments for Cartilage Repair: The Pivotal Role of MRI
In the rapidly evolving field of cartilage repair, three approaches are taking the lead: biological therapies, 3D printing and tissue engineering, and pharmacological interventions. Across these treatments, Magnetic Resonance Imaging (MRI) is proving indispensable for enhancing efficacy, safety, and personalization.
- Biological Therapies: Ensuring EfficacyBiological therapies like platelet-rich plasma (PRP) and mesenchymal stem cell (MSC) injections aim to use the bodys healing mechanisms. MRIs real-time tracking ensures that these agents accurately reach and integrate into the damaged cartilage. The dynamic monitoring allows clinicians to adapt treatment plans, making the therapies more effective and reducing the chances of failure.
- 3D Printing and Tissue Engineering. Precision and Personalization 3D printing and tissue engineering provide the exciting possibility of patient-specific implants. MRIs initial scans serve as blueprints, capturing the damaged cartilages intricate geometry, essential for accurate 3D printing. After implantation, MRI continues to be invaluable by assessing the implant’s integration with native tissues. This post-surgical evaluation is crucial for immediate intervention if complications arise, thus ensuring better long-term outcomes.
- Pharmacological Interventions.Safety and Accelerated Development MRI technology also plays a key role in the development and assessment of new pharmaceutical agents for cartilage repair, such as disease-modifying osteoarthritis drugs (DMOADs). High-resolution imaging offers clinicians quantitative data on cartilage health before and after treatment, critical for the success of clinical trials. Moreover, MRI can identify adverse effects early on, allowing for quick cessation or adjustment of the treatment. This accelerates the pace at which safer and more effective cartilage-repairing drugs are developed.
Challenges
Magnetic Resonance Imaging (MRI) has made remarkable contributions to the field of cartilage repair by providing unparalleled diagnostic capabilities. However, despite its groundbreaking impact, the technology faces significant challenges that can be categorized into three main areas: cost and accessibility, resolution and sensitivity, and standardization.
- Cost and Accessibility The financial burden associated with MRI scans is a major limitation. Scans can range from hundreds to thousands of dollars, depending on factors like the complexity of imaging and location of the healthcare facility. This makes MRI scans largely inaccessible for those without adequate insurance or those living in low-income regions. Furthermore, the lack of quality MRI facilities in rural areas exacerbates the issue of accessibility. Innovations like telemedicine, mobile MRI units, and public-private partnerships are making strides in reducing these barriers, yet much work remains to ensure universal accessibility.
- Resolution and Sensitivity Traditional MRI techniques often miss early-stage cartilage deterioration due to limitations in resolution and sensitivity. To address this, researchers are focusing on emerging technologies like Ultra-High Field (UHF) MRI and Quantitative Susceptibility Mapping (QSM). These offer unprecedented spatial resolution and enhanced signal-to-noise ratios. Additionally, machine learning algorithms are being utilized to analyze MRI data with heightened sensitivity. These advancements are promising but bring their own challenges, such as increased costs, longer scan times, and the need for specialized expertise.
- Standardization A significant obstacle in MRI cartilage assessment is the lack of standardized protocols, which affects the comparability and consistency of research studies. Organizations like the Radiological Society of North America (RSNA) and the American College of Radiology (ACR) are working on developing standardized guidelines for musculoskeletal imaging. The Quantitative Imaging Biomarkers Alliance (QIBA) is another initiative aiming to establish reproducible methods for cartilage evaluation. The incorporation of machine learning has the potential to further standardize MRI outputs across various systems, although this too comes with its own set of challenges.
Future Directions
In the future, the field of cartilage repair is poised for remarkable advancements:
1. Artificial Intelligence (AI): AI-driven image analysis can enhance the precision of cartilage assessments, aiding in early detection and personalized treatment planning.
- Superior Detection.Traditional imaging often falls short in capturing early signs of cartilage degradation, due to the limitations of human interpretation. AI algorithms, particularly those using deep learning, offer a solution by detecting complex patterns often missed by the human eye. This enables timely medical interventions, significantly boosting treatment success rates.
- Personalized Therapeutics.AIs utility extends beyond early detection to enabling personalized treatment plans. AI models can analyze unique cartilage characteristics and cross-reference them with extensive datasets, thereby recommending the most effective treatments, whether they be biological therapies, 3D-printed implants, or pharmacological interventions. This targeted approach increases the likelihood of successful patient outcomes.
2. Biomechanics and Functional Assessment are taking center stage as MRI techniques advance to include real-time evaluations of joint movements, offering a more nuanced understanding of cartilage health.
- Dynamic VisualizationTraditional MRI primarily provides static images, capturing a limited snapshot of ever-changing cartilage conditions. New MRI methods are shifting towards real-time functional imaging that visualizes cartilage and adjacent joint structures in action, thereby expanding our understanding of how cartilage performs under various physiological stresses.
- Quantifying Mechanical Properties This real-time capability also enables quantitative assessments of biomechanical properties, such as stiffness and elasticity. Understanding these parameters is crucial for devising more effective, personalized treatment plans.
- Clinical Applications Real-time functional MRI could be a game-changer in sports medicine, aiding athletes who frequently subject their joints to strenuous activities. It can also significantly impact surgical planning by offering insights into joint kinematics, enabling more accurate predictions of postoperative outcomes.
3. Drug Discovery is an emerging frontier in MRI’s utility, expanding its role from diagnostics to an invaluable player in high-throughput screening for cartilage repair agents.
- Targeted ScreeningMRI’s detailed imaging enables precise drug targeting by illuminating specific cartilage issues, such as degeneration or altered water content. This specificity allows for focused drug development, increasing the odds of discovering effective treatments.
- Efficacy and SafetyMRI’s real-time imaging capabilities offer unprecedented insights into drug interactions with cartilage. This feature accelerates adjustments to drug formulations and dosages, shortening the often-lengthy clinical trial process.
- Pharmacological InsightsMRI also sheds light on a drug’s pharmacodynamics and pharmacokinetics, informing dosage optimization and minimizing side effects, thus contributing to the drug development pipeline more holistically.
Conclusion
Magnetic Resonance Imaging has become a cornerstone in the field of cartilage repair, enabling non-invasive, detailed assessment of cartilage health and function.
As technological advances continue, MRI promises even greater precision in cartilage assessment. Its evolving synergy with emerging fields like AI and biomechanics is shaping a future where personalized and efficient treatments could become standard practice. While challenges in cost, accessibility, and expertise persist, they are surmountable as MRI moves into realms like high-throughput drug screening and real-time function assessments. As researchers continue to explore innovative treatments and technologies, MRI will play an increasingly crucial role in guiding treatment decisions, monitoring outcomes, and ultimately improving the quality of life for individuals suffering from cartilage-related joint conditions. The future of cartilage repair is bright, thanks to the powerful synergy between MRI and cutting-edge therapeutic strategies.
Visit ia-grp.com to discover how IAG’s advanced AI-powered technology platform DYNAMIKA and extensive experience in deploying AI in drug development can elevate your early and late phase trials. Explore the possibilities, seize the opportunities, and let the synergy of AI and medical imaging propel your biotech company into the future.
#AI #MedicalImaging #DrugDiscovery #PrecisionMedicine #ClinicalTrials #DrugRepurposing #Biotechnology #Healthcare #Innovation #Pharmaceuticals #PersonalizedMedicine #MedicalImageAnalysis #MRI #CT #Ultrasound #PET #PharmaceuticalInnovationv#Biotech #CEOv #AI-Powered #DrugDiscovery #Biotech #LifeSciences #Technology #osteoarthritis #MachineLearning #LifeSciences #BiotechInnovation #MedicalTech #FutureOfPharma
Reach out to our expert team, as you are designing and planning your trial. imaging.experts@ia-grp.com
About IAG, Image Analysis Group
IAG, Image Analysis Group is a strategic partner to bio-pharmaceutical companies developing new treatments to improve patients’ lives. Our dynamic Strategy, Trial Solutions and Bio-Partnering divisions work closely to meet critical needs of biotechnology companies: funding, clinical development, and monetization of their assets. We fuse decades of therapeutic insights, risk-sharing business model and agile culture to accelerate novel drug development. IAG broadly leverages its core imaging expertise, proprietary technology platform DYNAMIKA and capabilities to support an objective early go no/ go decision and drive excellence for tomorrow’s innovative therapeutic agents with speed.
