Fluorescence Imaging
Fluorescence imaging utilizes molecules with fluorescent property – fluorophores – that are excited by specific wavelengths of light, depending on the molecular property.
Subsequently, light is re-emitted with less energy but with longer wavelengths.
The penetration depth of the light depends on the wavelengths.
The near-infrared (NIR) spectrum of 700–1200 nm, the so-called “optical window,” has advantages for in vivo fluorescence imaging as there are less photon scattering, less absorption and less autofluorescence from tissue compared to light in the the visual spectrum. Whereas fluorescence in the visual spectrum can be detected by the human eye, fluorescence in the NIR spectrum requires a digital camera system as light in the NIR spectrum it is not visible to the human eye (Vonk et al, 2021).
Fluorescence guided surgery is an intraoperative medical technique used to generate a real-time fluorescence image to guide the surgical procedure for optimal resection of the tumor (Vonk et al, 2021 and Zheng et al, 2019).
About IAG, Image Analysis Group
IAG is a unique partner to life sciences companies developing new treatment and driving the hope of the up-coming precision medicine. IAG leverages expertise in medical imaging and the power of DYNAMIKA™, our proprietary cloud-based platform, to de-risk clinical development and deliver lifesaving therapies into the hands of patients much sooner. IAG provides early drug efficacy assessments, smart patient recruitment and predictive analysis of advanced treatment manifestations, thus lowering investment risk and accelerating study outcomes.
Acting as imaging Contract Research Organization, IAG’s experts also recognize the significance of a comprehensive approach to asset development. They actively engage in co-development projects with both private and public sectors, demonstrating a commitment to cultivating collaboration and advancing healthcare solutions.
Contact our expert team: imaging.experts@ia-grp.com
Experience: Scoring Systems
- RANO
- PET-based Standard Uptake Value (SUV)
- Volumetric Assessment
Experience: Imaging
- Fluorescence
- Anatomical CT / MRI
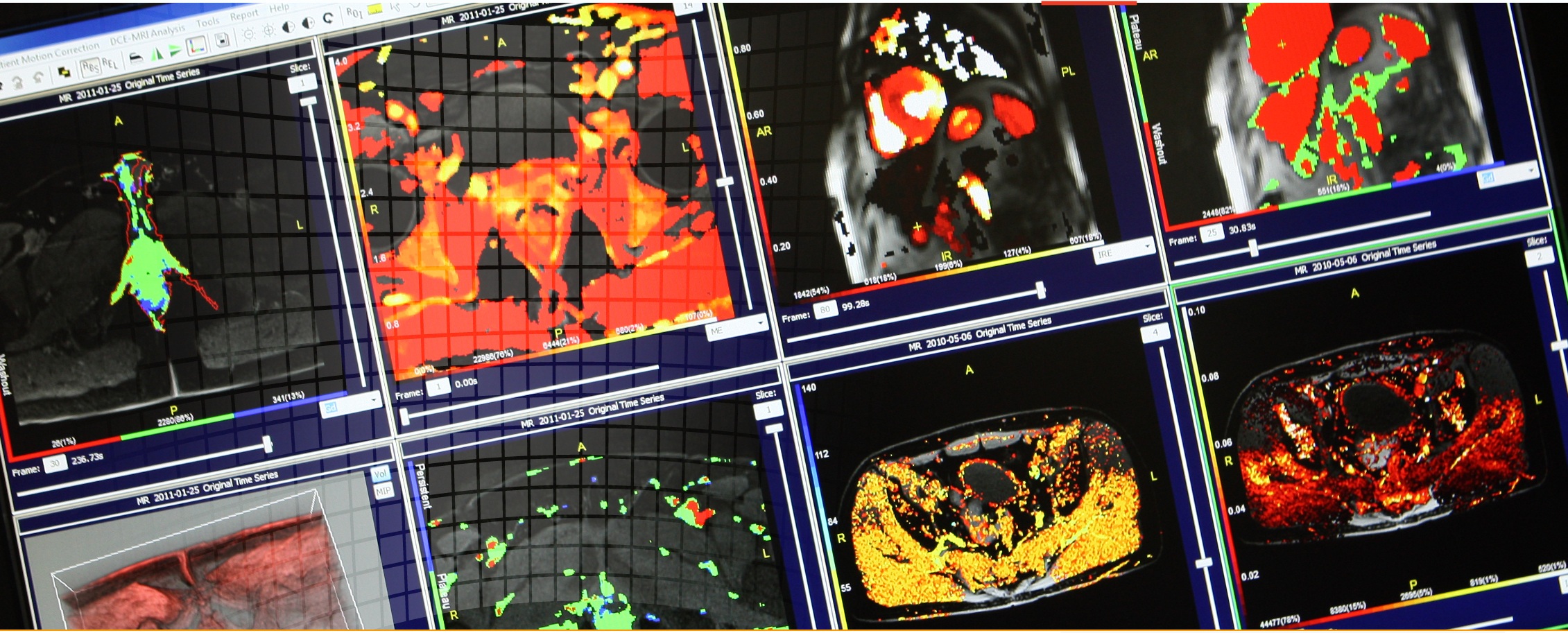
- perfusion imaging (DCE-MRI, DSC)
- diffusion imaging (ADC, DWI, DTI)
- Multiparametric MRI (mpMRI)
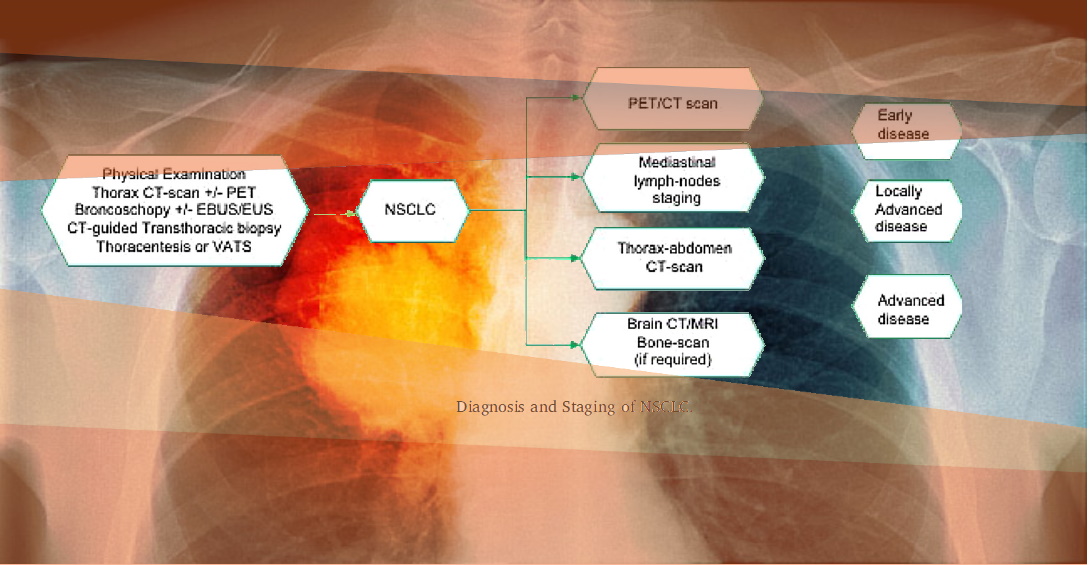
- PET/CT
- PET/MRI
- T-cell Labelling
- T-Cell Tracing
Publications
Since 2007, over 2000 articles were published to cover scientific discoveries, technology break-throughs and special cases. We list here some critically important papers and abstracts.
Testimonials
Combining our technologies and business advisory services with promising life science companies has yielded spectacular results over the past five years. As a trusted partner to many biotech and pharma companies, IAG’s team is proud to share your words and quotes.