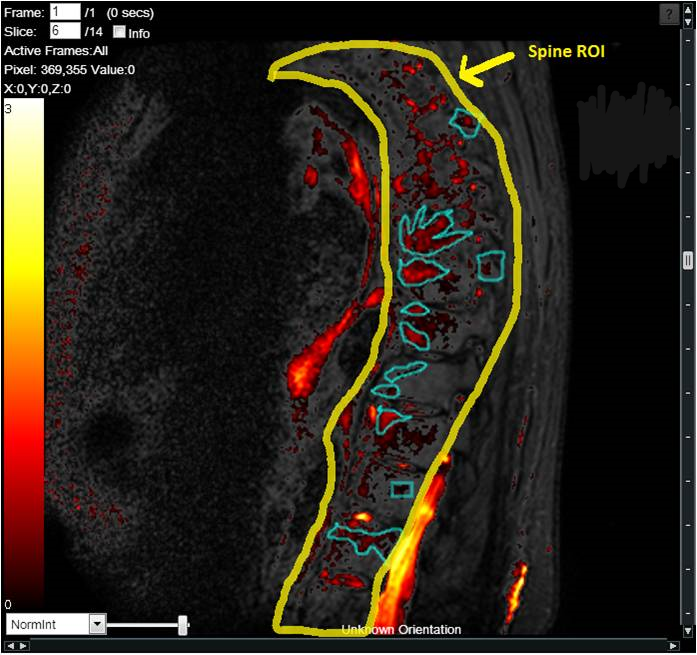
End-to-End Support of Osteoarthritis Studies
MAKE OUR EXPERT TEAM YOUR NEW PARTNERS
Accelerate Recruitment: Every day in drug development counts as patients are waiting for new treatments. IAG’s streamlined processes and global network expedite clinical trials without compromising quality.
Expertise You Can Trust: IAG has a proven track record of tackling complex imaging challenges – from finding the right managing sites, integrating novel endpoints, assessing drug safety and supporting M&A discussions.
Global Support, Local Focus: We placed our team of imaging specialists in geographically important hubs (EU, Asia, USA), which enables us to provide efficient answers to the sites and support global trials, with speed.
Value-Driven Partnerships: We prioritize delivering the highest quality data at competitive cost. We thrive to build long term relationships with our partners and clients.
IAG’S QUALITY & EFFICIENCY
We understand the value of time and transparency in clinical development, and power our trials with DYNAMIKA platform. It is designed to efficiently process imaging data, support all trial stakeholders, deliver results via reports or dashboards. Thus, helping our partners, readers and sponsors to fast decisions.
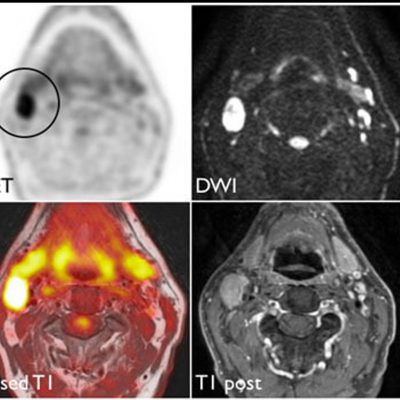
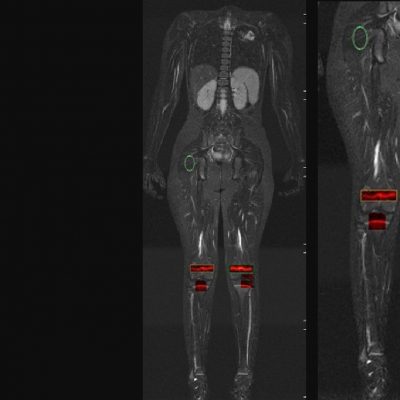
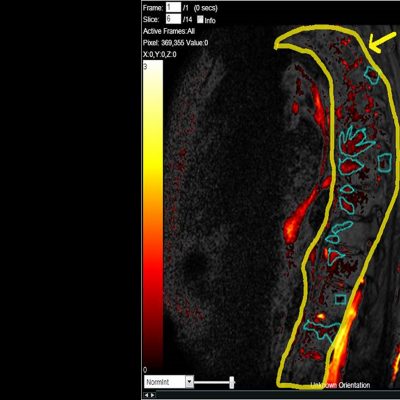
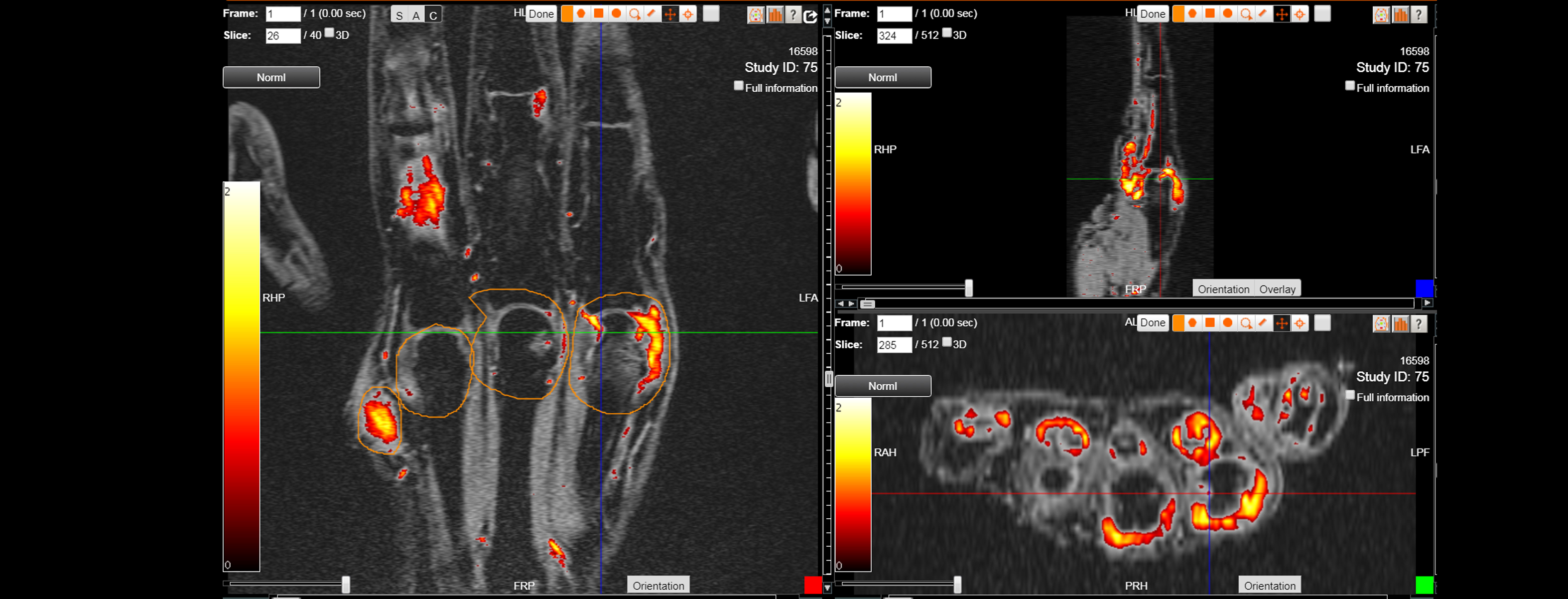
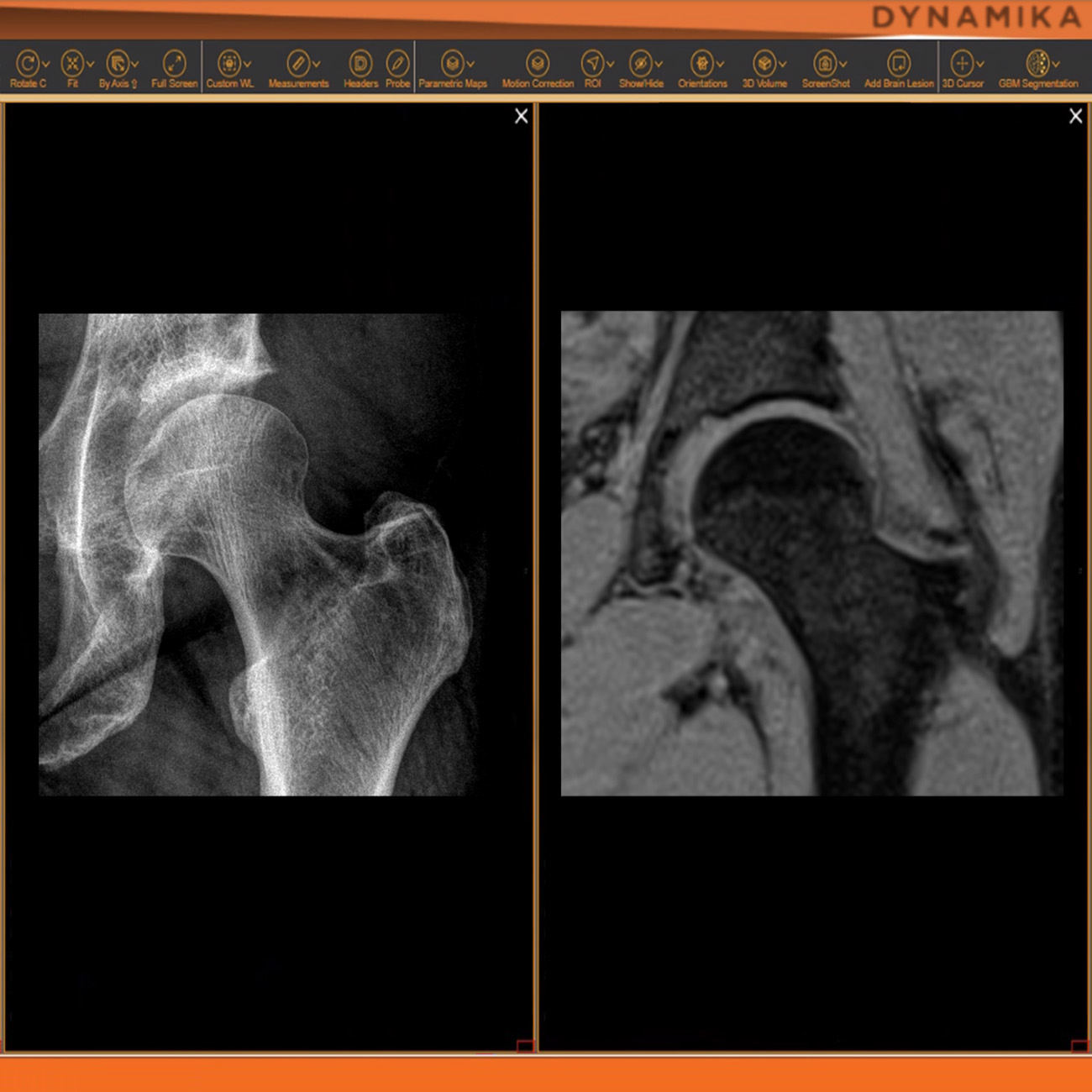
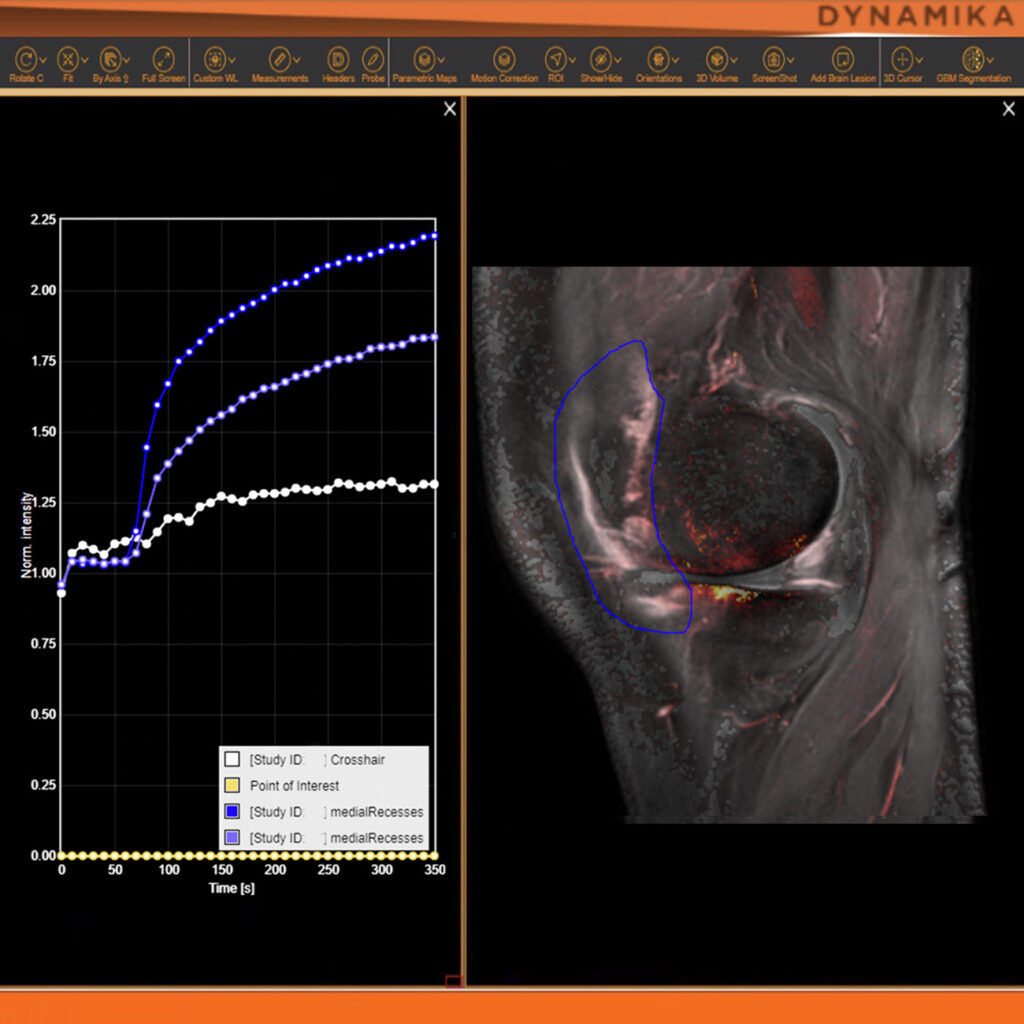
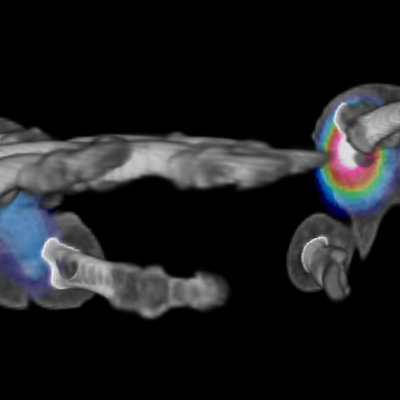
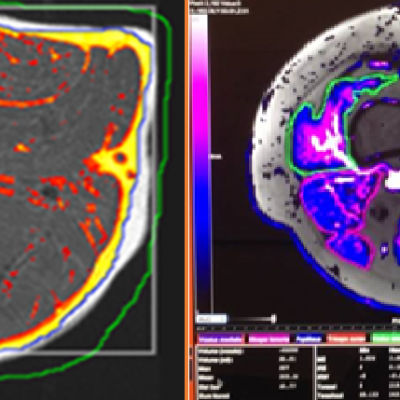
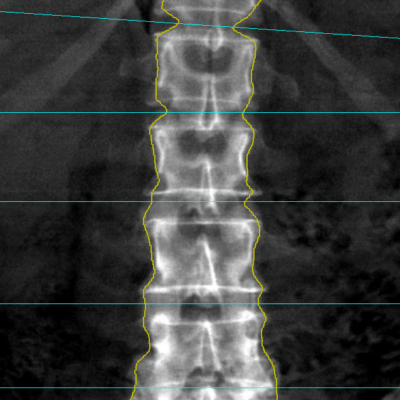
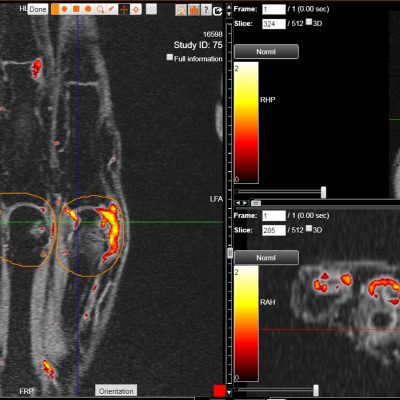
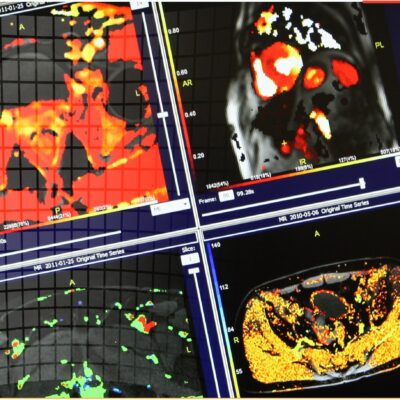
As an example, IAG was contacted by the USA based global pharmaceutical company to support their phase II clinical trial. The objective was to collect DCE-MRI scans from globally distributed sites and assess their drug for efficacy. Dynamic Contrast Enhanced MRI was used to assess the inflammatory changes.
Our sponsor has chosen us for our expertise in the field, our global network, and our proprietary platform where analysis was made in real-time, and the result was shared with the different parties.
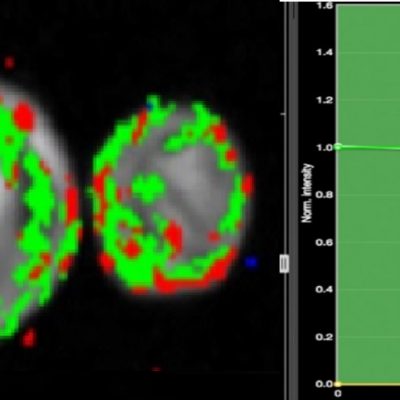
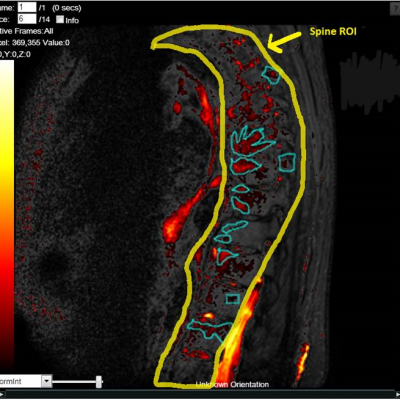
The work involving 60 patients, which were analysed with IAG’s proprietary analysis technique showing that in this trial of patients with osteoarthritis, significant improvement in sub-clinical, synovial-based inflammation after 6 months of therapy was detected with this novel MRI-based quantitative tool.
KEY TO OA STUDY SUCCESS
Accessibility: IAG has worked with many imaging facilities and can recommend high-performing Imaging Sites for your study, reducing risk and improving patient recruitment.
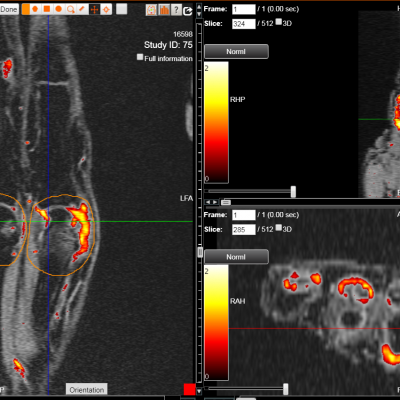
Transparency: DYNAMIKA is a proprietary, secure image transfer and processing system that provides real-time access to tracking information throughout the study.
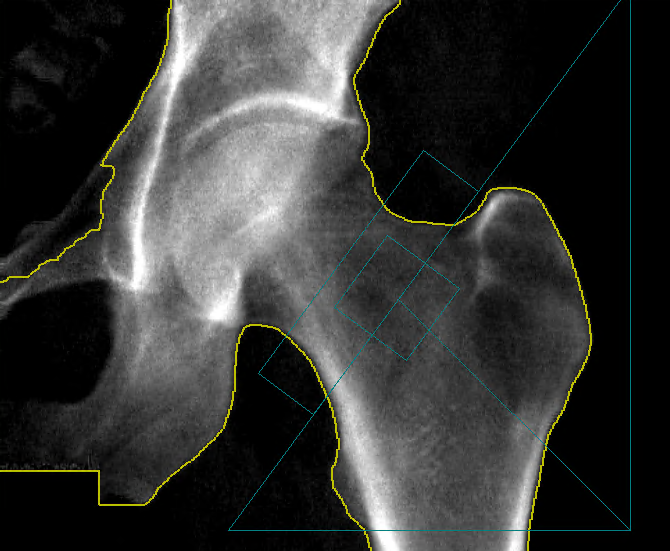
Speed: X-ray scans acquired at screening have to be read and adjudicated with speed. IAG provides reports to the sites within 1-5 days, allowing accelerated recruitment.
Disease Modification: When deploying advanced imaging (MR, PET, CT) to assess drug efficacy, we ensure that we can advice on the optimal endpoints and support regulatory discussions.
OUR PUBLICTAIONS
- Quantifying disease activity and damage by maging in rheumatoid arthritis and osteoarthritis
- Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in psoriatic arthritis (MIDAS): a cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis
- The effect of exercise therapy on inflammatory activity assessed by MRI in knee osteoarthritis: Secondary outcomes from a randomized controlled trial
- Osteoarthritis phenotypes and novel therapeutic targets
Would you be interested in receiving regular updates and
staying informed with the latest news?
We invite you to subscribe to our newsletter.
MULTIPLE SITES & ONE STUDY: SIMPLIFY THE DATA MANAGEMENT

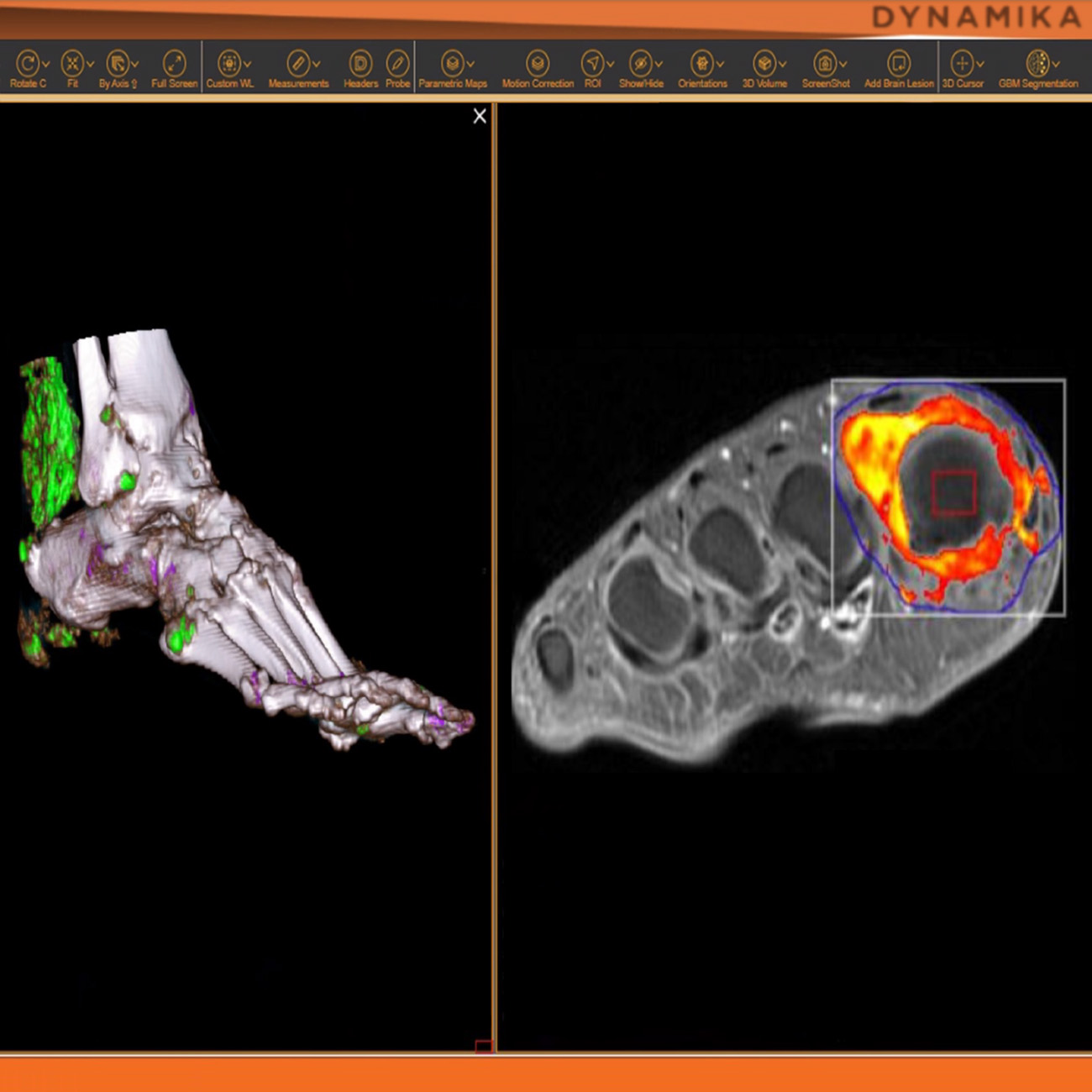
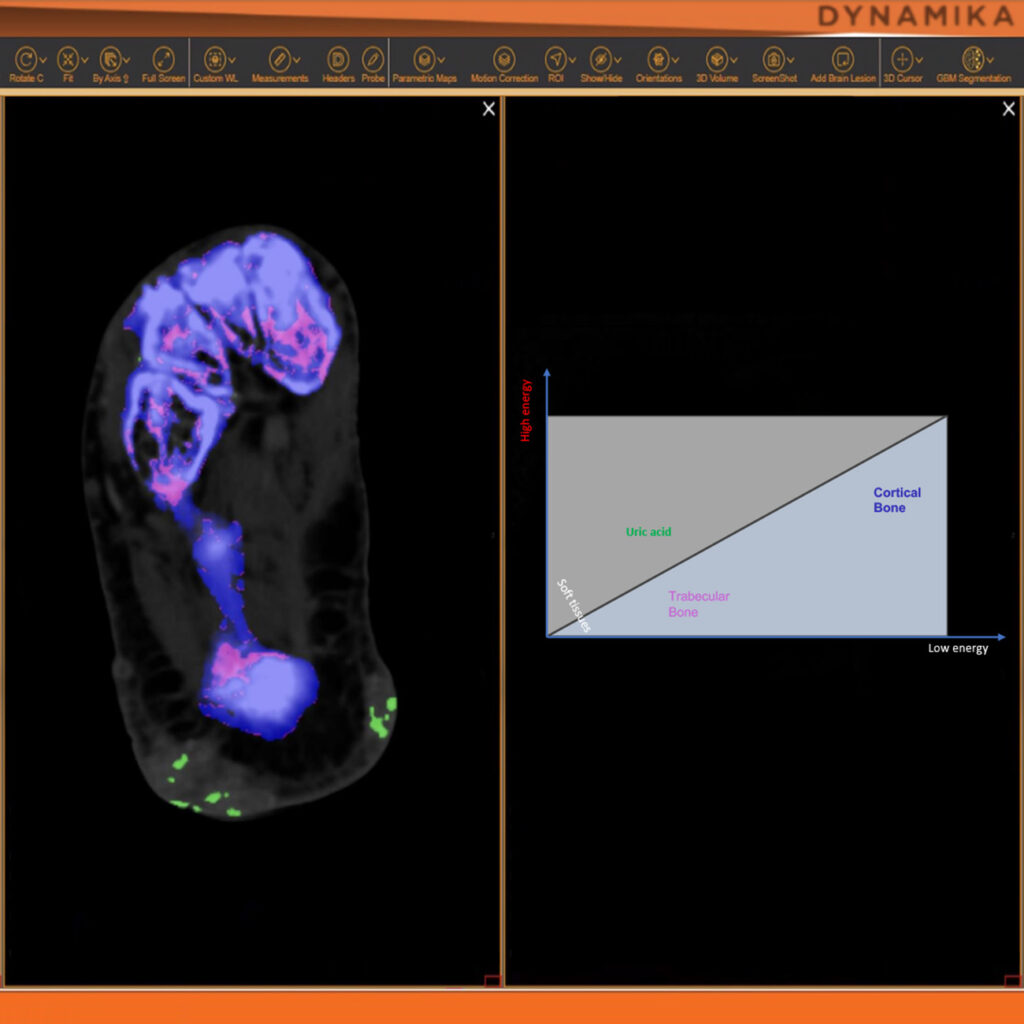
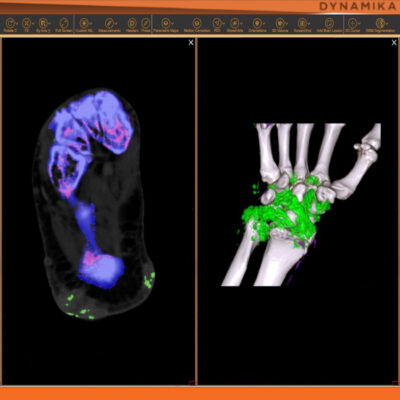
Managing data across multiple sites in clinical trials can be complex and time-consuming. Recognizing this challenge, our team of experts developed DYNAMIKA. It is IAG’s proprietary enterprise-scale cloud-based platform for imaging processing and data management. It works across multi-centre trial settings to enable central imaging review. This one comprehensive software system controls trial progress, conducts central reads and, when needed, it can be integrated with AI tools for earlier read-outs and decision support. Read more here.
MORE THAN A PARTNER, AN EXTENSION OF YOUR TEAM
Image Analysis Group is a unique partner to life sciences companies. Our goal is to accelerate novel drug developments, by using the right analytical tools and a modern trial infrastructure. At IAG, we are committed to helping our biotech and pharma partners to efficiently develop novel life-changing medicines, accelerating their R&D pipelines through advanced analytics, IAG’s technology solutions and imaging contract research services. We see each project as an oportunity to build a long lasting partnership and to become an extension of your team.































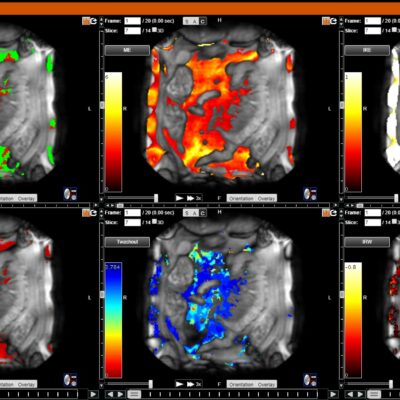
![RCC The image above is allustrating DCE MRI maps of a treatment responder pre-SABR (a-d) and post-SABR (e-h) including (left-right) IRE, AUC, Ktrans and Ve with tumour outlined in orange, generated with Dynamika [4]. The corresponding slice between pre-SABR and post-SABR scans.](https://www.ia-grp.com/wp-content/uploads/2017/09/RCC-400x377.jpg)